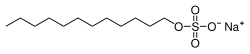
Sodium dodecyl sulfate
Its hydrocarbon tail combined with a polar "headgroup" give the compound amphiphilic properties that make it useful as a detergent.
[citation needed] It is a component in hand soap, toothpastes, shampoos, shaving creams, and bubble bath formulations, for its ability to create a foam (lather), for its surfactant properties, and in part for its thickening effect.
[10] SDS is used in cleaning procedures,[11] and is commonly used as a component for lysing cells during RNA extraction or DNA extraction, inhibiting the activity of nucleases, enzymes that can degrade DNA, protecting the integrity of the isolated genetic material, and for denaturing proteins in preparation for electrophoresis in the SDS-PAGE technique.
[13] In this way, the difference in mobility of the polypeptide chains in the gel can be attributed solely to their length as opposed to both their native charge and shape.
[25] For instance, SDS is a component, along with other chain-length amphiphiles, when produced from coconut oil, and is known as sodium coco sulfate (SCS).
[26] SDS is available commercially in powder, pellet, and other forms (each differing in rates of dissolution), as well as in aqueous solutions of varying concentrations.
[citation needed] It has been shown to irritate the skin of the face, with prolonged and constant exposure (more than an hour) in young adults.
[30][31][32] SDS is a common ingredient in toothpastes due to its low cost,[33] its lack of impact on taste,[33] and its desirable action as a foaming agent.
[34] Primary sources from the group of Irma Rantanen at University of Turku, Finland claim that SLS-containing pastes cause more dry mouth (xerostomia) than their proposed alternative.
However, a 2011 Cochrane review of these studies, and of the more general area, concludes that there "is no strong evidence... that any topical therapy is effective for relieving the symptom of dry mouth.