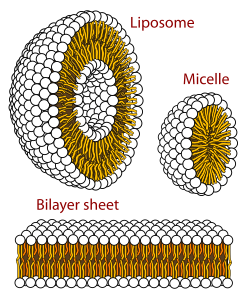
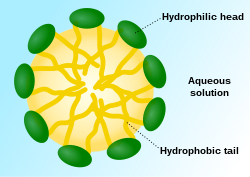
Micelle
As early as 1913, he postulated the existence of "colloidal ions" to explain the good electrolytic conductivity of sodium palmitate solutions.
[6] These highly mobile, spontaneously formed clusters came to be called micelles, a term borrowed from biology and popularized by G.S.
Micelles represent a molecular assembly, in which the individual components are thermodynamically in equilibrium with monomers of the same species in the surrounding medium.
However, the lipophilic "tails" of surfactant molecules have less contact with water when they are part of a micelle—this being the basis for the energetic drive for micelle formation.
In a micelle, the hydrophobic tails of several surfactant molecules assemble into an oil-like core, the most stable form of which having no contact with water.
By contrast, surfactant monomers are surrounded by water molecules that create a "cage" or solvation shell connected by hydrogen bonds.
The extent of lipid solubility is determined by the unfavorable entropy contribution due to the ordering of the water structure according to the hydrophobic effect.
In water, the hydrophobic effect is the driving force for micelle formation, despite the fact that assembling surfactant molecules is unfavorable in terms of both enthalpy and entropy of the system.
The micelle packing parameter equation is utilized to help "predict molecular self-assembly in surfactant solutions":[10] where
Moreover, thanks to the larger hydrophilic and hydrophobic parts, block copolymers can have a much more pronounced amphiphilic nature when compared to surfactant molecules.
Thanks to these two characteristics, a water solution of PS-PEO micelles of sufficiently high molecular weight can be considered kinetically frozen.
[16][17] Moreover, the stability against dilution and vast range of morphologies of kinetically frozen micelles make them particularly interesting, for example, for the development of long circulating drug delivery nanoparticles.
These inverse micelles are proportionally less likely to form on increasing headgroup charge, since hydrophilic sequestration would create highly unfavorable electrostatic interactions.
It is well established that for many surfactant/solvent systems a small fraction of the inverse micelles spontaneously acquire a net charge of +qe or -qe.
Supermicelles are formed via bottom-up chemical approaches, such as self-assembly of long cylindrical micelles into radial cross-, star- or dandelion-like patterns in a specially selected solvent; solid nanoparticles may be added to the solution to act as nucleation centers and form the central core of the supermicelle.
[20][21] When surfactants are present above the critical micelle concentration (CMC), they can act as emulsifiers that will allow a compound that is normally insoluble (in the solvent being used) to dissolve.
The most common example of this phenomenon is detergents, which clean poorly soluble lipophilic material (such as oils and waxes) that cannot be removed by water alone.
[26] Unlike conventional micellar catalysis,[27] the reactions occur solely on the charged micelles' surface.
Micelle formation is essential for the absorption of fat-soluble vitamins and complicated lipids within the human body.
This allows the absorption of complicated lipids (e.g., lecithin) and lipid-soluble vitamins (A, D, E, and K) within the micelle by the small intestine.
During the process of milk-clotting, proteases act on the soluble portion of caseins, κ-casein, thus originating an unstable micellar state that results in clot formation.