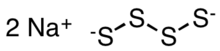Sodium tetrasulfide
[2] It is a precursor to some specialty polymers and intermediates in prototypes of the sodium-sulfur battery.
It is produced through the reaction between elemental sulfur and sodium hydrosulfide in alcoholic solution:[3] The polysulfide anions adopt zig-zag chains of sulfur atoms.
[4] Upon treatment with acid, it is converted to hydrogen sulfide and elemental sulfur.
In one commercial application, it is used to produce the cross-linking agent bis(triethoxysilylpropyl)tetrasulfide:[5] Sometimes as a mixture with other polysulfides, sodium tetrasulfide is used to produce the polymer called thiokol.
The reaction involves alkylation with ethylene chloride: These materials, which have the approximate formula (C2H4)Sx]n (x ~ 4), are highly resistant to degradation by solvents and acids.