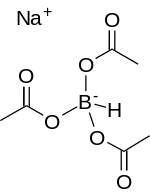
Sodium triacetoxyborohydride
Like other borohydrides, it is used as a reducing agent in organic synthesis.
[2][3][4] However, unlike sodium cyanoborohydride, the triacetoxyborohydride hydrolyzes readily, nor is it compatible with methanol.
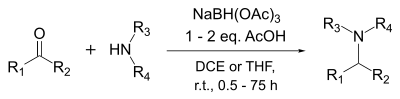
NaBH(OAc)3 may also be used for reductive alkylation of secondary amines with aldehyde-bisulfite adducts.
[5] The combination of Na[BH4] with carboxylic acids results in the formation of acyloxyborohydride species other than sodium triacetoxyborohydride.
These modified species can perform a variety of reductions not normally associated with borohydride chemistry, such as alcohols to hydrocarbons and nitriles to primary amines.