Reductive amination
It is a common method to make amines and is widely used in green chemistry since it can be done catalytically in one-pot under mild conditions.
Investigation into biocatalysts, such as imine reductases, have allowed for higher selectivity in the reduction of chiral amines which is an important factor in pharmaceutical synthesis.
[2] In a direct reaction, the carbonyl and amine starting materials and the reducing agent are combined and the reductions are done sequentially.
[2] The two most common methods for direct reductive amination are hydrogenation with catalytic platinum, palladium, or nickel catalysts and the use of hydride reducing agents like cyanoborohydride (NaBH3CN).
Its catalytic efficiency stems from the ability of palladium to adsorb hydrogen gas, forming active hydride species.
This reaction typically occurs under mild conditions with excellent selectivity, which often makes H2/Pd the first choice for synthesizing amines in pharmaceuticals and fine chemicals.
[3] Sodium cyanoborohydride (NaBH3CN) is soluble in hydroxylic solvents, stable in acidic solutions, and has different selectivities depending on the pH.
[7] For this reason, NaBH3CN is an ideal reducing agent for one-pot direct reductive amination reactions that don't isolate the intermediate imine.
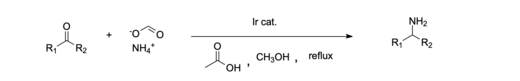
[20] Homogenous Iridium (III) catalysts have been shown to be effective in the reductive amination of carboxylic acids, which in the past has been more difficult than aldehydes and ketones.
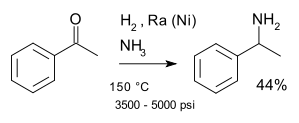
[15] In industry, tertiary amines such as triethylamine and diisopropylethylamine are formed directly from ketones with a gaseous mixture of ammonia and hydrogen and a suitable catalyst.
[21][22] Reductive amination can occur sequentially in one-pot reactions, which eliminates the need for intermediate purifications and reduces waste.
[1][26] In the critically acclaimed drama Breaking Bad, main character Walter White uses the reductive amination reaction to produce his high purity methamphetamine, relying on phenyl-2-propanone and methylamine.