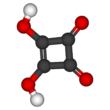
Squaric acid
Squaric acid is a reagent for chemical synthesis, used for instance to make photosensitive squaraine dyes and inhibitors of protein tyrosine phosphatases.
[6] Because the negative charges are equally distributed between each oxygen atom, the dianion of squaric acid is completely symmetrical (unlike squaric acid itself) with all C−C bond lengths identical and all C−O bond lengths identical.
[citation needed] Squaramides are prepared by displacement of alkoxy or chloride groups from C4O2X2 (X = OR, Cl).
[13] Squarate dianion behaves similarly to oxalate, forming mono- and polynuclear complexes with hard metal ions.
The water is bound to the cobalt atom, and the crystal structure consists of a cubic arrangement of hollow cells, whose walls are either six squarate anions (leaving a 7 Å wide void) or several water molecules (leaving a 5 Å void).
[14] Copper(II) squarate monomeric and dimeric mixed-ligand complexes were synthesized and characterized.
[16][4] Although impractical, squarate and related anions such as deltate C3O2−3 and acetylenediolate C2O2−2 are obtainable by reductive coupling of carbon monoxide using organouranium complexes.