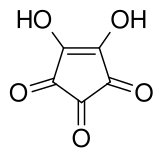
Croconic acid
[10] Infrared and Raman assignments indicate that the equalization of the carbon–carbon bond lengths, thus the electronic delocalization, follows with an increase in counter-ion size for salts.
[6] This result leads to a further interpretation that the degree of aromaticity is enhanced for salts as a function of the size of the counter-ion.
The same study provided quantum mechanical DFT calculations for the optimized structures and vibrational spectra which were in agreement with experimental findings.
The croconate anion forms hydrated crystalline coordination compounds with divalent cations of transition metals, with general formula M(C5O5)·3H2O; where M stands for copper (yielding a brown solid), iron (dark purple), zinc (yellow), nickel (green), manganese (dark green), or cobalt (purple).
These complexes all have the same orthorhombic crystal structure, consisting of chains of alternating croconate and metal ions.
[11] The croconate anion also forms compounds with trivalent cations such as aluminium (yellow), chromium (brown), and iron (purple).