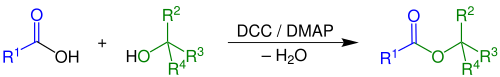Steglich esterification
[1] It is an adaptation of an older method for the formation of amides by means of DCC (dicyclohexylcarbodiimide) and 1-hydroxybenzotriazole (HOBT).
A characteristic is the formal uptake of water generated in the reaction by DCC, forming the urea compound dicyclohexylurea (DCU).
If the esterification is slow, a side-reaction occurs, diminishing the final yield or complicating purification of the product.
This side-reaction is a 1,3-rearrangement of the O-acyl intermediate to an N-acylurea which is unable to further react with the alcohol.
DMAP suppresses this side reaction, acting as an acyl transfer-reagent in the following manner: