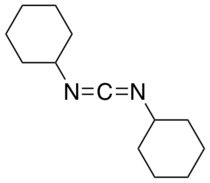
N,N'-Dicyclohexylcarbodiimide
[5] Yields of up to 67% have been achieved using this route: DCC has also been prepared from dicyclohexylurea using a phase transfer catalyst.
The majority of the DCU is thus readily removed by filtration, although the last traces can be difficult to eliminate from non-polar products.
[7] In protein synthesis (such as Fmoc solid-state synthesizers), the N-terminus is often used as the attachment site on which the amino acid monomers are added.
The negatively charged oxygen will act as a nucleophile, attacking the central carbon in DCC.
In combination with dimethyl sulfoxide (DMSO), DCC affects the Pfitzner-Moffatt oxidation.
[13] Thermal hazard analysis by differential scanning calorimetry (DSC) shows DCC poses minimal explosion risks.