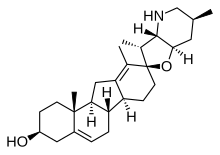
Cyclopamine
It is a teratogenic component of corn lily (Veratrum californicum), which when consumed during gestation has been demonstrated to induce birth defects, including the development of a single eye (cyclopia) in offspring.
[2] Later work suggested that differing rain patterns had changed grazing behaviours, which led to a greater quantity of corn lily to be ingested by pregnant sheep.
[3] Cyclopamine interrupts the sonic hedgehog signalling pathway, instrumental in early development, ultimately causing birth defects.
Cyclopamine was discovered as one of three steroidal alkaloids isolated from Veratrum californicum and was named after its effects on sheep embryos.
Four decades later, a team led by Professor Phillip Beachy linked the effect of cyclopamine to the sonic hedgehog gene.
Cyclopia was induced through silencing the sonic hedgehog gene, suggesting Cyclopamine acted through a similar mechanism.
[2] Cyclopamine consists of six rings, including a C-nor-D-homosteroid backbone linked to a octahydrofuro[3,2-b]pyridine system through a spirocentre.
[5] Veratramine is highly toxic, acting through excitation of the central nervous system causing seizures – similarly to serotonin.
[9] Vismodegib was designed to account for hydrogen bonding with the Smoothened receptor and to overcome the solubility issues of cyclopamine (through inclusion of the chlorine atom).