Antimony
Antimony compounds have been known since ancient times and were powdered for use as medicine and cosmetics, often known by the Arabic name kohl.
The most common applications for metallic antimony are in alloys with lead and tin, which have improved properties for solders, bullets, and plain bearings.
Coins of antimony were issued in China's Guizhou in 1931; durability was poor, and minting was soon discontinued because of its softness and toxicity.
It crystallises in a trigonal cell, isomorphic with bismuth and the gray allotrope of arsenic, and is formed when molten antimony is cooled slowly.
The trifluoride SbF3 is prepared by the reaction of Sb2O3 with HF:[32] It is Lewis acidic and readily accepts fluoride ions to form the complex anions SbF−4 and SbF2−5.
Antimony trioxide dissolves in concentrated acid to form oxoantimonyl compounds such as SbOCl and (SbO)2SO4.
Because stibine has a positive heat of formation, it is thermodynamically unstable and thus antimony does not react with hydrogen directly.
"[42] The British archaeologist Roger Moorey was unconvinced the artifact was indeed a vase, mentioning that Selimkhanov, after his analysis of the Tello object (published in 1975), "attempted to relate the metal to Transcaucasian natural antimony" (i.e. native metal) and that "the antimony objects from Transcaucasia are all small personal ornaments.
[42] The Roman scholar Pliny the Elder described several ways of preparing antimony sulfide for medical purposes in his treatise Natural History, around 77 AD.
[44] The Greek naturalist Pedanius Dioscorides mentioned that antimony sulfide could be roasted by heating by a current of air.
[43] Antimony was frequently described in alchemical manuscripts, including the Summa Perfectionis of Pseudo-Geber, written around the 14th century.
It was purported to be written by a Benedictine monk, writing under the name Basilius Valentinus in the 15th century; if it were authentic, which it is not, it would predate Biringuccio.
[43] With the advent of challenges to phlogiston theory, it was recognized that antimony is an element forming sulfides, oxides, and other compounds, as do other metals.
[50][51] The medieval Latin form, from which the modern languages and late Byzantine Greek take their names for antimony, is antimonium.
The popular etymology, from ἀντίμοναχός anti-monachos or French antimoine, would mean "monk-killer", which is explained by the fact that many early alchemists were monks, and some antimony compounds were poisonous.
[57]: 28 The standard chemical symbol for antimony (Sb) is credited to Jöns Jakob Berzelius, who derived the abbreviation from stibium.
[62] The Arabic word for the substance, as opposed to the cosmetic, can appear as إثمد ithmid, athmoud, othmod, or uthmod.
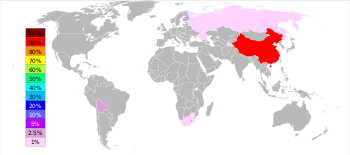
[64] In 2022, according to the US Geological Survey, China accounted for 54.5% of total antimony production, followed in second place by Russia with 18.2% and Tajikistan with 15.5%.
[67] Chinese production of antimony is expected to decline in the future as mines and smelters are closed down by the government as part of pollution control.
No significant antimony deposits in China have been developed for about ten years, and the remaining economic reserves are being rapidly depleted.
[69] For antimony-importing regions, such as Europe and the U.S., antimony is considered to be a critical mineral for industrial manufacturing that is at risk of supply chain disruption.
With global production coming mainly from China (74%), Tajikistan (8%), and Russia (4%), these sources are critical to supply.
[64] Antimony is mainly used as the trioxide for flame-proofing compounds, always in combination with halogenated flame retardants except in halogen-containing polymers.
[77] Markets for these flame-retardants include children's clothing, toys, aircraft, and automobile seat covers.
They are also added to polyester resins in fiberglass composites for such items as light aircraft engine covers.
Antimony is used in antifriction alloys (such as Babbitt metal),[81] in bullets and lead shot, electrical cable sheathing, type metal (for example, for linotype printing machines[82]), solder (some "lead-free" solders contain 5% Sb),[83] in pewter,[84] and in hardening alloys with low tin content in the manufacturing of organ pipes.
[64] In the 1990s antimony was increasingly being used in semiconductors as a dopant in n-type silicon wafers[87] for diodes, infrared detectors, and Hall-effect devices.
[113] The guidelines are: The tolerable daily intake (TDI) proposed by WHO is 6 μg antimony per kilogram of body weight.
[119] Occupational exposure may cause respiratory irritation, pneumoconiosis, antimony spots on the skin, gastrointestinal symptoms, and cardiac arrhythmias.
[118] Antimony toxicity typically occurs either due to occupational exposure, during therapy or from accidental ingestion.