Strain (chemistry)
In chemistry, a molecule experiences strain when its chemical structure undergoes some stress which raises its internal energy in comparison to a strain-free reference compound.
[1] This isn't always the case; if the difference in enthalpy is small, entropy can have a larger effect on the equilibrium.
[2] We find that the actual conformational distribution of butane is 70% anti and 30% gauche at room temperature.
Experimentally, strain energy is often determined using heats of combustion which is typically an easy experiment to perform.
First, one could compare to a similar compound that lacks strain, such as in the previous methylcyclohexane example.
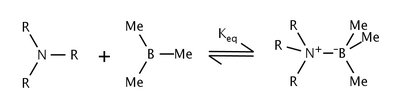
The effects of steric strain in the reaction of trialkylamines and trimethylboron were studied by Nobel laureate Herbert C. Brown et al.[6] They found that as the size of the alkyl groups on the amine were increased, the equilibrium constant decreased as well.
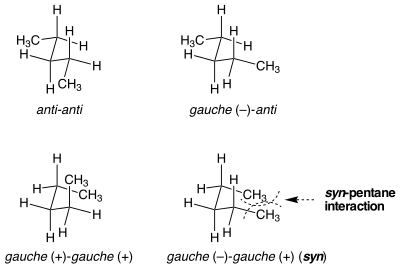
There are two different ways to put both of the bonds the central in n-pentane into a gauche conformation, one of which is 3 kcal mol−1 higher in energy than the other.
[1] When the two methyl-substituted bonds are rotated from anti to gauche in opposite directions, the molecule assumes a cyclopentane-like conformation where the two terminal methyl groups are brought into proximity.
These types of compounds usually take a more linear conformation to avoid the steric strain between the substituents.
Recent research has shown that the staggered conformation may be more stable due to a hyperconjugative effect.
[9] The simplest examples of angle strain are small cycloalkanes such as cyclopropane and cyclobutane, which are discussed below.
[10] Molecular mechanics or force field approaches allow to calculate such strain contributions, which then can be correlated e.g. with reaction rates or equilibria.
Another example is the solvolysis of bridgehead tosylates with steric energy differences between corresponding bromide derivatives (sp3) and the carbenium ion as sp2- model for the transition state.
[12] (Figure 2) In principle, angle strain can occur in acyclic compounds, but the phenomenon is rare.
[1] In comparison, smaller cycloalkanes are much higher in energy due to increased strain.
Cyclopropane is analogous to a triangle and thus has bond angles of 60°, much lower than the preferred 109.5° of an sp3 hybridized carbon.
Cyclobutane experiences similar strain, with bond angles of approximately 88° (it isn't completely planar) and eclipsed hydrogens.
In synthetic allosteric systems there are typically two or more conformers with stability differences due to strain contributions.
In addition, the response time of such allosteric switches depends on the strain of the conformer interconversion transitions state.