Allosteric regulation
In the fields of biochemistry and pharmacology an allosteric regulator (or allosteric modulator) is a substance that binds to a site on an enzyme or receptor distinct from the active site, resulting in a conformational change that alters the protein's activity, either enhancing or inhibiting its function.
Allosteric regulations are a natural example of control loops, such as feedback from downstream products or feedforward from upstream substrates.
The term allostery comes from the Ancient Greek allos (ἄλλος), "other", and stereos (στερεός), "solid (object)".
The term orthostery comes from the Ancient Greek orthós (ὀρθός) meaning “straight”, “upright”, “right” or “correct”.
For proteins in which subunits exist in more than two conformations, the allostery landscape model described by Cuendet, Weinstein, and LeVine,[6] can be used.
[8] A morpheein is a homo-oligomeric structure that can exist as an ensemble of physiologically significant and functionally different alternate quaternary assemblies.
The allostery landscape model introduced by Cuendet, Weinstein, and LeVine[6] allows for the domains to have any number of states and the contribution of a specific molecular interaction to a given allosteric coupling can be estimated using a rigorous set of rules.
Molecular dynamics simulations can be used to estimate a system's statistical ensemble so that it can be analyzed with the allostery landscape model.
An example of this model is seen with the Mycobacterium tuberculosis, a bacterium that is perfectly suited to adapt to living in the macrophages of humans.
Another example of allosteric activation is seen in cytosolic IMP-GMP specific 5'-nucleotidase II (cN-II), where the affinity for substrate GMP increases upon GTP binding at the dimer interface.
Glycine is a major post-synaptic inhibitory neurotransmitter in mammalian spinal cord and brain stem.
Phosphofructokinase (generally referred to as PFK) is an enzyme that catalyses the third step of glycolysis: the phosphorylation of fructose-6-phosphate into fructose 1,6-bisphosphate.
This change causes its affinity for substrate (fructose-6-phosphate and ATP) at the active site to decrease, and the enzyme is deemed inactive.
Non-regulatory allostery could comprise any other ions besides sodium (calcium, magnesium, zinc), as well as other chemicals and possibly vitamins.
Diazepam is a positive allosteric modulator at the benzodiazepine regulatory site, and its antidote flumazenil is a receptor antagonist.
More recent examples of drugs that allosterically modulate their targets include the calcium-mimicking cinacalcet and the HIV treatment maraviroc.
There are a number of advantages in using allosteric modulators as preferred therapeutic agents over classic orthosteric ligands.
[21] If an allosteric modulator does not possess appreciable efficacy, it can provide another powerful therapeutic advantage over orthosteric ligands, namely the ability to selectively tune up or down tissue responses only when the endogenous agonist is present.
[21] Oligomer-specific small molecule binding sites are drug targets for medically relevant morpheeins.
[26] In many multivalent supramolecular systems[27] direct interaction between bound ligands can occur, which can lead to large cooperativities.
Due to the often high receptor selectivity and lower target-based toxicity, allosteric regulation is also expected to play an increasing role in drug discovery and bioengineering.
Each protein is annotated with detailed description of allostery, biological process and related diseases, and each modulator with binding affinity, physicochemical properties and therapeutic area.
[31][32][33][34][35][36][37][38] In part, this growing interest is a result of their general importance in protein science, but also because allosteric residues may be exploited in biomedical contexts.
Allosteric sites at the surface generally play regulatory roles that are fundamentally distinct from those within the interior; surface residues may serve as receptors or effector sites in allosteric signal transmission, whereas those within the interior may act to transmit such signals.

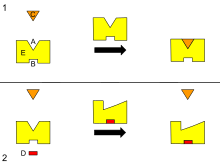
B – Allosteric site
C – Substrate
D – Inhibitor
E – Enzyme
This is a diagram of allosteric regulation of an enzyme.