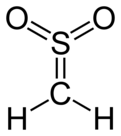
Sulfene
It is the simplest member of the sulfenes, the group of compounds which are S,S-dioxides of thioaldehydes and thioketones, and have the general formula R2C=SO2.
[1][2][3] The first general method for preparation of sulfene as an intermediate, reported simultaneously in 1962 by Gilbert Stork[4] and by Günther Optiz,[5] involved the removal of hydrogen chloride from methanesulfonyl chloride using triethylamine in the presence of an enamine as trapping agent.
A simple alternative which avoids the use of amines involves desilylation of trimethylsilylmethanesulfonyl chloride with cesium fluoride in the presence of trapping agents.
Sulfenes react with enamines, ynamines, and 1,3-cyclopentadienes to give thietanes, thietes and Diels-Alder adducts, respectively.
In the presence of a chiral tertiary amine complex, several sulfenes could be trapped with trichloroacetaldehyde (chloral) in a catalytic asymmetric synthesis of β-sulfones (four-membered ring sulfonate esters).