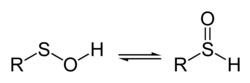Sulfenic acid
[5] Sulfenic acids are produced by the enzymatic decomposition of alliin and related compounds following tissue damage to garlic, onions, and other plants of the genus Allium.
1-Propenesulfenic acid, formed when onions are cut, is rapidly rearranged by a second enzyme, the lachrymatory factor synthase, giving syn-propanethial-S-oxide.
[7] Mass spectrometry with a DART ion source were used to identify 2-propenesulfenic formed when garlic is cut or crushed and to demonstrate that this sulfenic acid has a lifetime of less than one second.
[8] The pharmacological activity of certain drugs, such as omeprazole, esomeprazole, ticlopidine, clopidogrel, and prasugrel is proposed to involve sulfenic acid intermediates.
[12] Sulfoxides can undergo thermal elimination via an Ei mechanism to yield vinyl alkenes and sulfenic acids:[13][14] Compounds which react in this manner are used as polymer stabilizers where they protects against long term heat ageing,[15] structures based on thiodipropionate esters are popular.