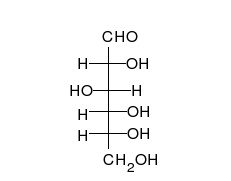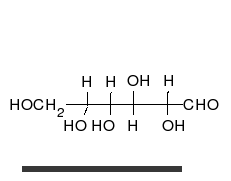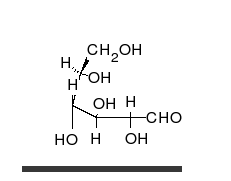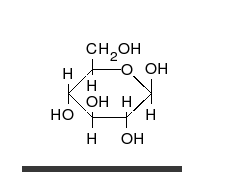
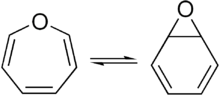
Tautomer
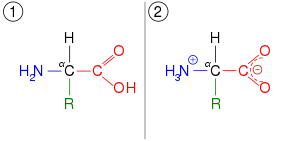
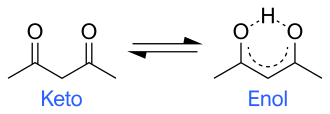
Tautomers are distinct chemical species that can be distinguished by their differing atomic connectivities, molecular geometries, and physicochemical and spectroscopic properties,[6] whereas resonance forms are merely alternative Lewis structure (valence bond theory) depictions of a single chemical species, whose true structure is a quantum superposition, essentially the "average" of the idealized, hypothetical geometries implied by these resonance forms.
[9][10] Other examples of this type of tautomerism can be found in bullvalene, and in open and closed forms of certain heterocycles, such as organic azides and tetrazoles,[11] or mesoionic münchnone and acylamino ketene.
For example, samples of 2-pyridone and 2-hydroxypyridine do not exist as separate isolatable materials: the two tautomeric forms are interconvertible and the proportion of each depends on factors such as temperature, solvent, and additional substituents attached to the main ring.
[8][13] Historically, each form of the substance was entered into databases such as those maintained by the Chemical Abstracts Service and given separate CAS Registry Numbers.
The facility to automatically recognise such potential tautomerism and ensure that all tautomers are indexed together has been greatly facilitated by the creation of the International Chemical Identifier (InChI) and associated software.