Aromatic sulfonation
To drive the equilibrium, dehydrating agents such as thionyl chloride can be added:[2] Historically, mercurous sulfate has been used to catalyze the reaction.
Due to their electron withdrawing effects, sulfonate protecting groups can be used to prevent electrophilic aromatic substitution.
They can also be installed as directing groups to affect the position where a substitution may take place.
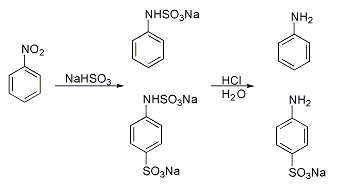
A classic named reaction is the Piria reaction (Raffaele Piria, 1851) in which nitrobenzene is treated with a metal bisulfite forming an aminosulfonic acid as a result of combined nitro group reduction and sulfonation.
[2][5][6] In the Tyrer sulfonation process (1917),[7] at some time of technological importance, benzene vapor is led through a vessel containing 90% sulfuric acid the temperature of which is increased from 100 to 180°C.