Sulfur
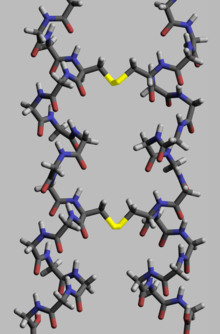
Disulfides, S–S bonds, confer mechanical strength and insolubility of the (among others) protein keratin, found in outer skin, hair, and feathers.
[c] Between its melting and boiling temperatures, octasulfur changes its allotrope again, turning from β-octasulfur to γ-sulfur, again accompanied by a lower density but increased viscosity due to the formation of polymers.
[19] Sulfur is insoluble in water but soluble in carbon disulfide and, to a lesser extent, in other nonpolar organic solvents, such as benzene and toluene.
Applying catalysts and/or supply of external energy may vary sulfur's oxidation state and the composition of reaction products.
The long coiled polymeric molecules make the brownish substance elastic, and in bulk it has the feel of crude rubber.
The preponderance of 32S is explained by its production in the so-called alpha-process (one of the main classes of nuclear fusion reactions) in exploding stars.
Other stable sulfur isotopes are produced in the bypass processes related with 34Ar, and their composition depends on a type of a stellar explosion.
The δ13C and δ34S of coexisting carbonate minerals and sulfides can be used to determine the pH and oxygen fugacity of the ore-bearing fluid during ore formation.
[39] The distinctive colors of Jupiter's volcanic moon Io are attributed to various forms of molten, solid, and gaseous sulfur.
[45][46] Significant deposits in salt domes occur along the coast of the Gulf of Mexico, and in evaporites in eastern Europe and western Asia.
Fossil-based sulfur deposits from salt domes were once the basis for commercial production in the United States, Russia, Turkmenistan, and Ukraine.
English translations of the Christian Bible commonly referred to burning sulfur as "brimstone", giving rise to the term "fire-and-brimstone" sermons, in which listeners are reminded of the fate of eternal damnation that await the unbelieving and unrepentant.
[63] Indian alchemists, practitioners of the "science of chemicals" (Sanskrit: रसशास्त्र, romanized: rasaśāstra), wrote extensively about the use of sulfur in alchemical operations with mercury, from the eighth century AD onwards.
In traditional skin treatment, elemental sulfur was used (mainly in creams) to alleviate such conditions as scabies, ringworm, psoriasis, eczema, and acne.
[75] Molten sulfur is sometimes still used for setting steel bolts into drilled concrete holes where high shock resistance is desired for floor-mounted equipment attachment points.
The true Ancient Greek word for sulfur, θεῖον, theîon (from earlier θέειον, théeion), is the source of the international chemical prefix thio-.
[77] On the other hand, sulfur was the form eventually chosen in the United States, though multiple place names (such as White Sulphur Springs) use -ph-.
[82] In volcanic regions in Sicily, in ancient times, it was found on the surface of the Earth, and the "Sicilian process" was used: sulfur deposits were piled and stacked in brick kilns built on sloping hillsides, with airspaces between them.
Eventually the surface-borne deposits played out, and miners excavated veins that ultimately dotted the Sicilian landscape with labyrinthine mines.
"[83] Sulfur is still mined from surface deposits in poorer nations with volcanoes, such as Indonesia, and problems with working conditions still exist.
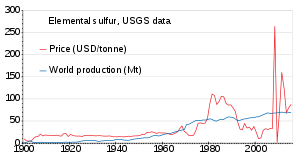
[85][86] Since then, sulfur has typically been produced from petroleum, natural gas, and related fossil resources, from which it is obtained mainly as hydrogen sulfide.
Amino acids synthesized by living organisms such as methionine and cysteine contain organosulfur groups (thioester and thiol respectively).
However, in some circumstances, soils can be depleted in sulfate, e.g. if this later is leached by meteoric water (rain) or if the requirements in sulfur for some types of crops are high.
Elemental sulfur (ES) is sometimes mixed with bentonite to amend depleted soils for crops with high requirement in organo-sulfur.
[91] Biologically produced sulfur particles are naturally hydrophilic due to a biopolymer coating and are easier to disperse over the land in a spray of diluted slurry, resulting in a faster uptake by plants.
In February 2022, researchers at Drexel University have not only created a prototypical battery that lasted 4000 recharge cycles, but also found the first monoclinic gamma sulfur that remained stable below 95 degrees Celsius.
[107] Primitive bacteria that live around deep ocean volcanic vents oxidize hydrogen sulfide for their nutrition, as discovered by Robert Ballard.
Many important cellular enzymes use prosthetic groups ending with -SH moieties to handle reactions involving acyl-containing biochemicals: two common examples from basic metabolism are coenzyme A and alpha-lipoic acid.
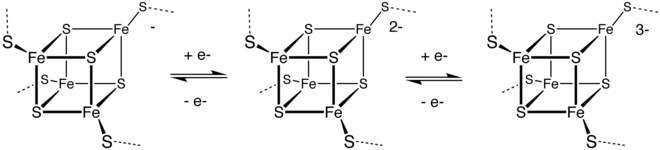
[126] Metalloproteins—in which the active site is a transition metal ion (or metal-sulfide cluster) often coordinated by sulfur atoms of cysteine residues[127]—are essential components of enzymes involved in electron transfer processes.
[144] However, its presence in ambient air at concentration over 100–150 ppm quickly deadens the sense of smell,[145] and a victim may breathe increasing quantities without noticing until severe symptoms cause death.








3 )