Surface energy
They are found to be highly dynamic regions, which readily rearrange or react, so that energy is often reduced by such processes as passivation or adsorption.
[3] The most commonly used method is OWRK, which requires the use of two probe liquids and gives out as a result the total surface energy as well as divides it into polar and dispersive components.
At such temperatures the solid creeps and even though the surface area changes, the volume remains approximately constant.
While this is only strictly true for amorphous solids (glass) and liquids, isotropy is a good approximation for many other materials.
In the case of single-crystal materials, such as natural gemstones, anisotropy in the surface energy leads to faceting.
The shape of the crystal (assuming equilibrium growth conditions) is related to the surface energy by the Wulff construction.
To guarantee this, we need to create the slab carefully to make sure that the upper and lower surfaces are of the same type.
During sublimation of a substance, intermolecular forces between molecules are broken, resulting in a change in the material from solid to gas.
The following equation can be used as a reasonable estimate for surface energy: The presence of an interface influences generally all thermodynamic parameters of a system.
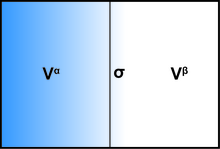
In order to demonstrate the thermodynamics of an interfacial system using the Gibbs model, the system can be divided into three parts: two immiscible liquids with volumes Vα and Vβ and an infinitesimally thin boundary layer known as the Gibbs dividing plane (σ) separating these two volumes.
Some examples include internal energy U, the number of molecules of the ith substance ni, and the entropy S. While these quantities can vary between each component, the sum within the system remains constant.
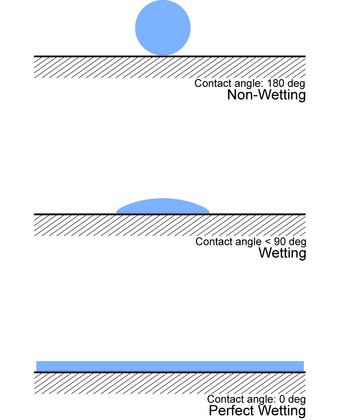
It is beneficial to define a new term interfacial excess Γi which allows us to describe the number of molecules per unit area: Surface energy comes into play in wetting phenomena.
[13] The most commonly used surface modification protocols are plasma activation, wet chemical treatment, including grafting, and thin-film coating.
The Kelvin equation is based on thermodynamic principles and is used to describe changes in vapor pressure caused by liquids with curved surfaces.
[21] This natural attraction is largely attributed to the powerful short-range van der Waals forces, as an effect of their surface energies.
A surface that is easy to wet is desirable when formulating a coating that requires good adhesion and appearance.
Finally, the particles are subjected to a repulsive force in order to keep them separated from one another and lowers the likelihood of flocculation.
Alternatively, steric or entropic repulsion is a phenomenon used to describe the repelling effect when adsorbed layers of material (such as polymer molecules swollen with solvent) are present on the surface of the pigment particles in dispersion.
Only certain portions (anchors) of the polymer molecules are adsorbed, with their corresponding loops and tails extending out into the solution.
[23] This crowding effect is accompanied by a decrease in entropy, whereby the number of conformations possible for the polymer molecules is reduced in the adsorbed layer.
As a result, energy is increased and often gives rise to repulsive forces that aid in keeping the particles separated from each other.