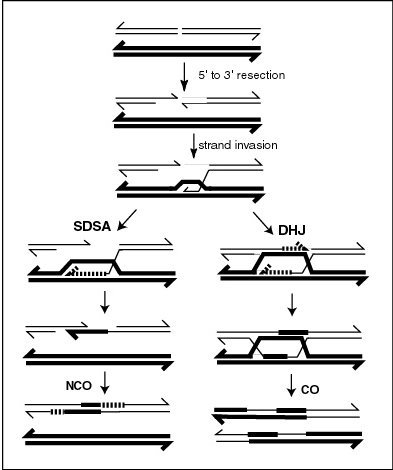
Synthesis-dependent strand annealing
Synthesis-dependent strand annealing (SDSA) is a major mechanism of homology-directed repair of DNA double-strand breaks (DSBs).
[6] In yeast, the D-loop is formed by strand invasion with the help of proteins Rad51 and Rad52,[7] and is then acted on by DNA helicase Srs2 to prevent formation of the double Holliday junction in order for the SDSA pathway to occur.
Therefore, although SDSA produces non-crossover products because flanking markers of heteroduplex DNA are not exchanged, gene conversion may occur, wherein nonreciprocal genetic transfer takes place between two homologous sequences.
Research in Drosophila melanogaster identified the Bloom syndrome helicase (Blm) as the enzyme promoting dissassembly of the D-loop.
[17] Sgs1(BLM) may disassemble D-loop structures analogous to early strand invasion intermediates and thus promote NCO formation by SDSA.
[17] The Sgs1 helicase forms a conserved complex with the topoisomerase III (Top3)-RMI1 heterodimer (that catalyzes DNA single strand passage).