Tanning (leather)
Historically, vegetable based tanning used tannin, an acidic chemical compound derived from the bark of certain trees, in the production of leather.
Tanning hide into leather involves a process which permanently alters the protein structure of skin, making it more durable and less susceptible to decomposition and coloring.
Ancient civilizations used leather for waterskins, bags, harnesses and tack, boats, armour, quivers, scabbards, boots, and sandals.
In some variations of the process, cedar oil, alum, or tannin was applied to the skin as a tanning agent.
The moisture content of hides and skins is greatly reduced, and osmotic pressure increased, to the point that bacteria are unable to grow.
They include, in order, soaking, liming, removal of extraneous tissues (unhairing, scudding and fleshing), deliming, bating or puering, drenching, and pickling.
The isoelectric point of the collagen (a tissue-strengthening protein unrelated to keratin) in the hide is also shifted to around pH 4.7 due to liming.
Any hairs remaining after liming are removed mechanically by scraping the skin with a dull knife, a process known as scudding.
In modern tanning, these enzymes are purified agents, and the process no longer requires bacterial fermentation (as from dung-water soaking) to produce them.
Mineral tanned leather is used principally for shoes, car seats, and upholstery in homes (sofas, etc.).
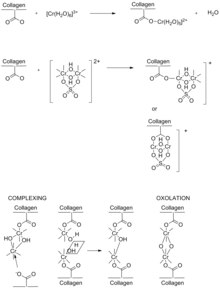
Chromium(III) sulfate ([Cr(H2O)6]2(SO4)3) has long been regarded as the most efficient and effective tanning agent.
During basification step of tanning, the carboxyl groups are ionized and coordinate as ligands to the chromium(III) centers of the oxo-hydroxide clusters.
Before the introduction of the basic chromium species in tanning, several steps are required to produce a tannable hide.
Once the desired level of penetration of chrome into the substance is achieved, the pH of the material is raised again to facilitate the process.
[17] Chromium's ability to form such stable bridged bonds explains why it is considered one of the most effective tanning compounds.
[13] Vegetable tanning uses tannins (a class of polyphenol astringent chemicals), which occur naturally in the bark and leaves of many plants.
Tannins bind to the collagen proteins in the hide and coat them, causing them to become less water-soluble and more resistant to bacterial attack.
In Ethiopia, the combined vegetable oils of Niger seed (Guizotia abyssinica) and flaxseeds were used in treating the flesh side of the leather, as a means of tawing, rather than of tanning.
Wet white can be produced using aldehydes, aluminum, zirconium, titanium, or iron salts, or a combination thereof.
Concerns with the toxicity and environmental impact of any chromium (VI) that may form during the tanning process have led to increased research into more efficient wet white methods.
[19] The use of alum alone for tanning rawhides is not recommended, as it shrinks the surface area of the skin, making it thicker and hard to the touch.
[20] Depending on the finish desired, the leather may be waxed, rolled, lubricated, injected with oil, split, shaved, or dyed.
This hexavalent chromium runoff and scraps are then consumed by animals, in the case of Bangladesh, chickens (the nation's most common source of protein).
Up to 25% of the chickens in Bangladesh contained harmful levels of hexavalent chromium, adding to the national health problem load.
Anthracene, which is used as a leather tanning agent, can cause problems in the kidneys and liver and is also considered a carcinogen.
Formaldehyde and arsenic, which are used for leather finishing, cause health problems in the eyes, lungs, liver, kidneys, skin, and lymphatic system and are also considered carcinogens.