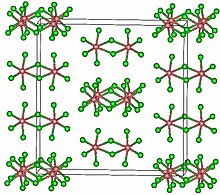
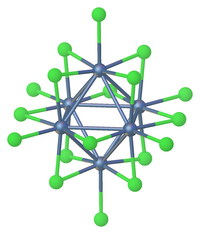
Tantalum(V) chloride
It takes the form of a white powder and is commonly used as a starting material in tantalum chemistry.
[3] The solubility of tantalum pentachloride increases slightly for the following series of aromatic hydrocarbons: This is reflected in the deepening of colour of the solutions from pale yellow to orange.
[5] TaCl5 forms stable complexes with ethers: TaCl5 also reacts with phosphorus pentachloride and phosphorus oxychloride, the former as a chloride donor and the latter serves as a ligand, binding through the oxygen: Tantalum pentachloride reacts with tertiary amines to give crystalline adducts.
Similarly TaCl5 reacts with lithium methoxide in anhydrous methanol to form related methoxy derivatives: Ammonia will displace most of the chloride ligands from TaCl5 to give a cluster.
Chloride is displaced more slowly by primary or secondary amines but the replacement of all five chloride centers by amido groups has been achieved by the use of lithium dialkylamides, as illustrated by the synthesis of pentakis(dimethylamido)tantalum: With alcohols, the pentachloride reacts to give alkoxides.