Technetium hexafluoride
The fact that the boiling point of the hexafluorides of uranium and technetium are very close to each other presents a problem in using fluoride volatility in nuclear reprocessing.
Below this temperature (measured at −19 °C), the solid structure is orthorhombic space group Pnma.
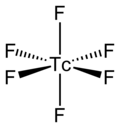
[2] The TcF6 molecule itself (the form important for the liquid or gas phase) has octahedral molecular geometry, which has point group (Oh).
[8] Preliminary measurements of magnetic moment yield a value of 0.45 μB, which is lower than expected for a d1 octahedral compound.
[9] TcF6 reacts with alkaline chlorides in iodine pentafluoride (IF5) solution to form hexafluorotechnetates.