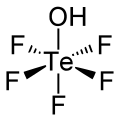
Teflic acid
Teflic acid is a chemical compound with the formula HOTeF5.
Teflic acid has a slightly distorted octahedral molecular geometry.
Teflic acid was accidentally discovered by Engelbrecht and Sladky.
Their synthesis did not yield the anticipated telluryl fluoride TeO2F2, but a mixture of volatile telluric compounds, containing HOTeF5:[2] Teflic acid can also be prepared from fluorosulfonic acid and barium tellurate:[3] It is also the first hydrolysis product of tellurium hexafluoride: The conjugate base of teflic acid is called the teflate anion, F5TeO− (not to be confused with triflate).
This property has allowed the preparation several highly unusual species such as the hexateflates M(OTeF5)−6 (in which M = As, Sb, Bi).