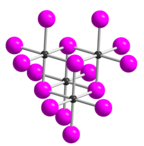
Tellurium tetraiodide
[2] In TeI4 the Te atoms are octahedrally coordinated and edges of the octahedra are shared.
[2] Tellurium tetraiodide can be prepared by reacting Te and iodomethane, CH3I.
[2] In the vapour TeI4 dissociates:[3] It can be also obtained by reacting telluric acid with hydrogen iodide.
[6] It is stable even in moist air and decomposes when heated, releasing iodine.
In solvents with donor properties such as acetonitrile, CH3CN ionic complexes are formed which make the solution conducting:[3] Five modifications of tellurium tetraiodide are known, all of which are composed of tetrameric molecules.