tert-Butanesulfinamide
[1][2][3] tert-Butanesulfinamide and the associated synthetic methodology was introduced in 1997 by Jonathan A. Ellman et al.[4] Enantiopure tert-butanesulfinamide can be prepared by enantioselective oxidation of inexpensive di-tert-butyl disulfide to the thiosulfinate followed by disulfide bond cleavage by lithium amide.
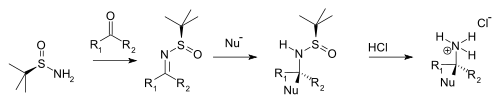
Condensation with ketones and aldehydes yields the corresponding N-tert-butanesulfinyl aldimines and ketimines.
On addition of hydrochloric acid the tert-butanesulfinyl group is removed, forming the chiral primary ammonium salt or amine (from aldehyde precursor) or the chiral secondary amine (ketone precursor).
Chiral sulfinimines as intermediates for the asymmetric synthesis of amines have also been developed by Franklin A.
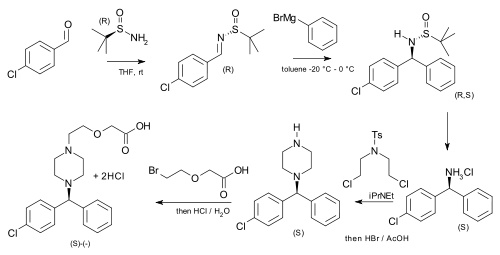
[5] tert-Butanesulfinamide has been used as an auxiliary in an asymmetric synthesis of cetirizine (more potent than the racemic mixture of the drug) starting from p-chlorobenzaldehyde and phenylmagnesium bromide.