Ammonium
To further confirm ammonia, it is passed through a glass rod dipped in an HCl solution (hydrochloric acid), creating white dense fumes of ammonium chloride.
Ammonia or ammonium ion when added to Nessler's reagent gives a brown color precipitate known as the iodide of Million's base in basic medium.
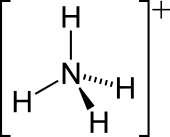
The lone electron pair on the nitrogen atom (N) in ammonia, represented as a line above the N, forms a coordinate bond with a proton (H+).
Depending on the number of organic groups, the ammonium cation is called a primary, secondary, tertiary, or quaternary.
An unusual class of organic ammonium salts is derivatives of amine radical cations, [•NR3]+ such as tris(4-bromophenyl)ammoniumyl hexachloroantimonate.
Because nitrogen often limits net primary production due to its use in enzymes that mediate the biochemical reactions that are necessary for life, ammonium is utilized by some microbes and plants.
[6] The amount of ammonium in soil that is available for nitrification by microbes varies depending on environmental conditions.
[7][8] For example, ammonium is deposited as a waste product from some animals, although it is converted into urea in mammals, sharks, and amphibians, and into uric acid in birds, reptiles, and terrestrial snails.
[12] For example, ammonium mobilization is one of the key factors for the symbiotic association between plants and fungi, called mycorrhizae.
[16] Because net primary production is often limited by nitrogen, increased ammonium levels could impact the biological communities that rely on it.
[18][19][20] Ammonium is expected to behave as a metal ([NH4]+ ions in a sea of electrons) at very high pressures, such as inside giant planets such as Uranus and Neptune.