Polyethylene terephthalate
Polyethylene terephthalate (or poly(ethylene terephthalate), PET, PETE, or the obsolete PETP or PET-P), is the most common thermoplastic polymer resin of the polyester family and is used in fibres for clothing, containers for liquids and foods, and thermoforming for manufacturing, and in combination with glass fibre for engineering resins.
"[8] Depending on its processing and thermal history, polyethylene terephthalate may exist both as an amorphous (transparent) and as a semi-crystalline polymer.
Polyester fibres are used in fashion apparel often blended with cotton, as heat insulation layers in thermal wear, sportswear and workwear and automotive upholstery.
PET sandwiches an additional polyvinyl alcohol (PVOH) or polyamide (PA) layer to further reduce its oxygen permeability.
These can be injection moulded into parts such as housings, covers, electrical appliance components and elements of the ignition system.
[16] PET is stoichiometrically a mixture of carbon and H2O, and therefore has been used in an experiment involving laser-driven shock compression which created nanodiamonds and superionic water.
[17][18] PET was patented in 1941 by John Rex Whinfield, James Tennant Dickson and their employer the Calico Printers' Association of Manchester, England.
E. I. DuPont de Nemours in Delaware, United States, first produced Dacron (PET fiber) in 1950 and used the trademark Mylar (boPET film) in June 1951 and received registration of it in 1952.
[29] In the Soviet Union, PET was first manufactured in the laboratories of the Institute of High-Molecular Compounds of the USSR Academy of Sciences in 1949, and its name "Lavsan" is an acronym thereof (лаборатории Института высокомолекулярных соединений Академии наук СССР).
[clarification needed] Transparent products can be produced by rapidly cooling molten polymer below the glass transition temperature (Tg) to form a non-crystalline amorphous solid.
[34] Like glass, amorphous PET forms when its molecules are not given enough time to arrange themselves in an orderly, crystalline fashion as the melt is cooled.
Light tends to scatter as it crosses the boundaries between crystallites and the amorphous regions between them, causing the resulting solid to be translucent.
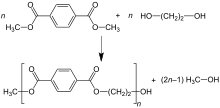
[41] The reactions can be summarized as follows: In the terephthalic acid process, MEG and PTA are esterified directly at moderate pressure (2.7–5.5 bar) and high temperature (220–260 °C).
A better process based on oxidation of ethanol has been proposed,[45] and it is also technically possible to make PTA from readily available bio-based furfural.
In the second step, the preforms are heated rapidly and then inflated against a two-part mold to form them into the final shape of the bottle.
Preforms (uninflated bottles) are now also used as robust and unique containers themselves; besides novelty candy, some Red Cross chapters distribute them as part of the Vial of Life program to homeowners to store medical history for emergency responders.
The preforms can be transported and stored by the thousand in a much smaller space than would finished containers, for the second stage to be carried out on the user site on a 'just in time' basis.
Its greatest merit is the reduction in space, product handling and energy, and far higher visual quality than can be achieved by the two-step system.
If the moisture level is too high, hydrolysis will reduce the molecular weight by chain scission, resulting in brittleness.
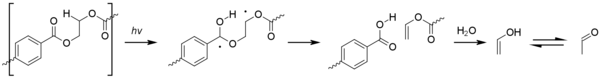
[48] When acetaldehyde is produced, some of it remains dissolved in the walls of a container and then diffuses into the product stored inside, altering the taste and aroma.
[49] Commentary published in Environmental Health Perspectives in April 2010 suggested that PET might yield endocrine disruptors under conditions of common use and recommended research on this topic.
An article published in Journal of Environmental Monitoring in April 2012 concludes that antimony concentration in deionized water stored in PET bottles stays within EU's acceptable limit even if stored briefly at temperatures up to 60 °C (140 °F), while bottled contents (water or soft drinks) may occasionally exceed the EU limit after less than a year of storage at room temperature.
[55] Fruit juice concentrates (for which no guidelines are established), however, that were produced and bottled in PET in the UK were found to contain up to 44.7 μg/L of antimony, well above the EU limits for tap water of 5 μg/L.
Microplastics which are present on the bottom of the river or seabed can be ingested by small marine life, thus entering the food chain.
PET microfibers generated by apparel wear, washing or machine drying can become airborne, and be dispersed into fields, where they are ingested by livestock or plants and end up in the human food supply.
[67] PET, like many plastics, is also an excellent candidate for thermal disposal (incineration), as it is composed of carbon, hydrogen, and oxygen, with only trace amounts of catalyst elements (but no sulfur).
The process involves holding a mixture of PET, water, nitric acid, and ethanol at a high temperature and pressure for eight hours, followed by centrifugation and drying.
[78] PET is also a desirable fuel for waste-to-energy plants, as it has a high calorific value which helps to reduce the use of primary resources for energy generation.
[81] Japanese scientists have isolated another bacterium, Ideonella sakaiensis, that possesses two enzymes which can break down the PET into smaller pieces digestible by the bacteria.
[84][85][86] Also, an enzyme based on a natural PET-ase was designed with the help of a machine learning algorithm to be able to tolerate pH and temperature changes by the University of Texas at Austin.