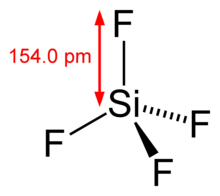
Silicon tetrafluoride
It was first prepared in 1771 by Carl Wilhelm Scheele by dissolving silica in hydrofluoric acid,[5] and later synthesized by John Davy in 1812.
[10] The hydrofluoric acid and silicon dioxide (SiO2) react to produce hexafluorosilicic acid:[10] In the laboratory, the compound is prepared by heating barium hexafluorosilicate (Ba[SiF6]) above 300 °C (572 °F) whereupon the solid releases volatile SiF4, leaving a residue of BaF2.
Alternatively, sodium hexafluorosilicate (Na2[SiF6]) may also be thermally decomposed at 400 °C (752 °F)—600 °C (1,112 °F) (optionally in inert nitrogen gas atmosphere) [11]: 8 This volatile compound finds limited use in microelectronics and organic synthesis.
[7] Staying in the 1980s, as part of the Low-Cost Solar Array Project by Jet Propulsion Laboratory,[13] it was investigated as a potentially cheap feedstock for polycrystalline silicon production in fluidized bed reactors.
[16] In 2001 it was listed by New Jersey authorities as a hazardous substance that is corrosive and may severely irritate or even burn skin and eyes.