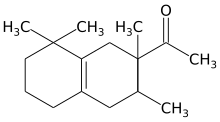
Tetramethyl acetyloctahydronaphthalenes
[6] Carrying out the initial Diels–Alder reaction using a Lewis acid catalyst such as aluminum chloride appears to ensure that the acetyl group is at position 2 of the resulting cyclohexene adduct, which distinguished Iso E Super from other (previously patented) fragrances based on tetramethylacetyloctaline.
[9] No data were available regarding chemical disposition, metabolism, or toxicokinetics; acute, short-term, subchronic, or chronic toxicity; synergistic or antagonistic activity; reproductive or teratological effects; carcinogenicity; genotoxicity; or immunotoxicity.
[5] The International Fragrance Association (IFRA) has published safe use levels for Iso E Super in consumer products.
OTNE has been detected in surface water at levels of 29–180 ng/L,[15][16] These values are well below the Predicted No Effect Concentration (PNEC) and as a result the overall environmental risk ratio (also referred to as RCR or PEC/PNECS) is determined to be below 1.
[7] In 2014 the US National Toxicology Program (NTP) conducted a 13-week repeat dose toxicity study and found no adverse effects.
[17] OTNE has been recommended for inclusion in an update for the EU Fragrance Allergens labelling for cosmetic products based on a small number of positive reactions in dermatological clinics of around 0.2% to 1.7% of patients tested in three studies[18] If the proposed SCCS Opinion is taken forward into legislation then OTNE will be labelled on cosmetic products in the EU, several years after publication of a new legislation.