Tetrasulfur tetranitride
It is a precursor to many S-N compounds and has attracted wide interest for its unusual structure and bonding.
When the properties of atoms are so highly similar, they often form extensive families of covalently bonded structures and compounds.
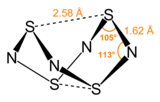
The pairs of sulfur atoms across the ring are separated by 2.586 Å, resulting in a cage-like structure as determined by single crystal X-ray diffraction.
[3] The nature of the transannular S–S interactions remains a matter of investigation because it is significantly shorter than the sum of the van der Waals radii[4] but has been explained in the context of molecular orbital theory.
S4N4 is thermochromic, changing from pale yellow below −30 °C to orange at room temperature to deep red above 100 °C.
[1] S4N4 was first prepared in 1835 by M. Gregory by the reaction of disulfur dichloride with ammonia,[8] a process that has been optimized:[9] Coproducts of this reaction include heptasulfur imide (S7NH) and elemental sulfur, and the latter equilibrates with more S4N4 and ammonium sulfide:[10] A related synthesis employs [NH4]Cl instead:[1] An alternative synthesis entails the use of (((CH3)3Si)2N)2S as a precursor with pre-formed S–N bonds.
The (((CH3)3Si)2N)2S reacts with the combination of SCl2 and SO2Cl2 to form S4N4, trimethylsilyl chloride, and sulfur dioxide:[11] S4N4 is a Lewis base at nitrogen.
[14] Electron-poor alkynes attack S4N4 to give a different cycloadduct of stoichiometry RC(NS)2SCR′.