Thermodynamics
His most important paper, "On the Moving Force of Heat",[3] published in 1850, first stated the second law of thermodynamics.
In 1909, Constantin Carathéodory presented a purely mathematical approach in an axiomatic formulation, a description often referred to as geometrical thermodynamics.
Properties can be combined to express internal energy and thermodynamic potentials, which are useful for determining conditions for equilibrium and spontaneous processes.
Non-equilibrium thermodynamics is often treated as an extension of the classical treatment, but statistical mechanics has brought many advances to that field.
By watching the valve rhythmically move up and down, Papin conceived of the idea of a piston and a cylinder engine.
[12] The first thermodynamic textbook was written in 1859 by William Rankine, originally trained as a physicist and a civil and mechanical engineering professor at the University of Glasgow.
The foundations of statistical thermodynamics were set out by physicists such as James Clerk Maxwell, Ludwig Boltzmann, Max Planck, Rudolf Clausius and J. Willard Gibbs.
Clausius, who first stated the basic ideas of the second law in his paper "On the Moving Force of Heat",[3] published in 1850, and is called "one of the founding fathers of thermodynamics",[14] introduced the concept of entropy in 1865.
During the years 1873–76 the American mathematical physicist Josiah Willard Gibbs published a series of three papers, the most famous being On the Equilibrium of Heterogeneous Substances,[15] in which he showed how thermodynamic processes, including chemical reactions, could be graphically analyzed, by studying the energy, entropy, volume, temperature and pressure of the thermodynamic system in such a manner, one can determine if a process would occur spontaneously.
[17] During the early 20th century, chemists such as Gilbert N. Lewis, Merle Randall,[18] and E. A. Guggenheim[19][20] applied the mathematical methods of Gibbs to the analysis of chemical processes.
[25][26] In 1854, the noun thermo-dynamics is used by Thomson and William Rankine to represent the science of generalized heat engines.
The qualifier classical reflects the fact that it represents the first level of understanding of the subject as it developed in the 19th century and describes the changes of a system in terms of macroscopic empirical (large scale, and measurable) parameters.
This field relates the microscopic properties of individual atoms and molecules to the macroscopic, bulk properties of materials that can be observed on the human scale, thereby explaining classical thermodynamics as a natural result of statistics, classical mechanics, and quantum theory at the microscopic level.
The term 'thermodynamic equilibrium' indicates a state of balance, in which all macroscopic flows are zero; in the case of the simplest systems or bodies, their intensive properties are homogeneous, and their pressures are perpendicular to their boundaries.
In an equilibrium state there are no unbalanced potentials, or driving forces, between macroscopically distinct parts of the system.
Thermodynamics is principally based on a set of four laws which are universally valid when applied to systems that fall within the constraints implied by each.
In the various theoretical descriptions of thermodynamics these laws may be expressed in seemingly differing forms, but the most prominent formulations are the following.
This statement implies that thermal equilibrium is an equivalence relation on the set of thermodynamic systems under consideration.
Systems are said to be in equilibrium if the small, random exchanges between them (e.g. Brownian motion) do not lead to a net change in energy.
Thus, if one seeks to decide whether two bodies are at the same temperature, it is not necessary to bring them into contact and measure any changes of their observable properties in time.
The first law of thermodynamics states: In a process without transfer of matter, the change in internal energy,
The second law refers to a system of matter and radiation, initially with inhomogeneities in temperature, pressure, chemical potential, and other intensive properties, that are due to internal 'constraints', or impermeable rigid walls, within it, or to externally imposed forces.
The many versions of the second law all express the general irreversibility of the transitions involved in systems approaching thermodynamic equilibrium.
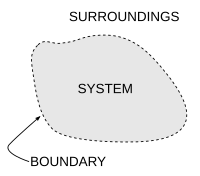
Transfers of energy as work, or as heat, or of matter, between the system and the surroundings, take place through the walls, according to their respective permeabilities.
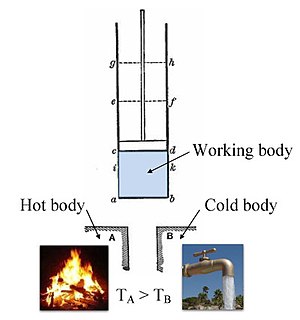
For example, in an engine, a fixed boundary means the piston is locked at its position, within which a constant volume process might occur.
State may be thought of as the instantaneous quantitative description of a system with a set number of variables held constant.
Although pressure is defined mechanically, a pressure-measuring device, called a barometer may also be constructed from a sample of an ideal gas held at a constant temperature.
In mechanics, for example, energy transfer equals the product of the force applied to a body and the resulting displacement.
The common conjugate variables are: Thermodynamic potentials are different quantitative measures of the stored energy in a system.
[37][38] In this formulation, thermodynamic concepts such as heat, entropy, and temperature are derived from quantities that are more directly measurable.