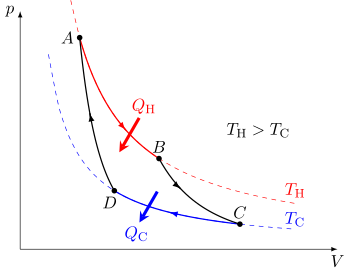
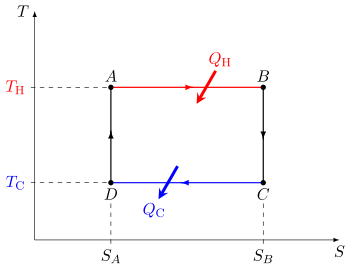
Carnot heat engine
The Carnot engine model was graphically expanded by Benoît Paul Émile Clapeyron in 1834 and mathematically explored by Rudolf Clausius in 1857, work that led to the fundamental thermodynamic concept of entropy.
[3] The efficiency depends only upon the absolute temperatures of the hot and cold heat reservoirs between which it operates.
In the process of going through this cycle, the system may perform work on its surroundings, thereby acting as a heat engine.
In the adjacent diagram, from Carnot's 1824 work, Reflections on the Motive Power of Fire,[4] there are "two bodies A and B, kept each at a constant temperature, that of A being higher than that of B.
These two bodies to which we can give, or from which we can remove the heat without causing their temperatures to vary, exercise the functions of two unlimited reservoirs of caloric.
The previous image shows the original piston-and-cylinder diagram used by Carnot in discussing his ideal engine.
Although in those early years, engines came in a number of configurations, typically QH was supplied by a boiler, wherein water was boiled over a furnace; QC was typically removed by a stream of cold flowing water in the form of a condenser located on a separate part of the engine.
This means the total entropy of system and surroundings (the entropies of the hot furnace, the "working fluid" of the heat engine, and the cold sink) remains constant when the "working fluid" completes one cycle and returns to its original state.
(In the general and more realistic case of an irreversible process, the total entropy of this combined system would increase.)
Thus, it implies that the total entropy change of the furnace and sink is zero, for the process to be reversible and the efficiency of the engine to be maximum.
The working fluid is brought back to its initial state after one cycle, and thus the change of entropy of the fluid system is 0, but the sum of the entropy changes in the hot and cold reservoir in this one cyclical process is greater than 0.
So, one would get: Similarly, at the time of heat injection from the fluid to the cold reservoir one would have, for the magnitude of total entropy change
Diesel knew a Carnot engine is an ideal that cannot be built, but he thought he had invented a working approximation.
Diesel's patented solution was: having achieved the highest temperature just by compressing the air, to add a small amount of fuel at a controlled rate, such that heating caused by burning the fuel would be counteracted by cooling caused by air expansion as the piston moved.
Hence all the heat from the fuel would be transformed into work during the isothermal expansion, as required by Carnot's theorem.
He calculated that, were he to reduce the peak pressure to a less ambitious 90 atmospheres, he would sacrifice only 5% of the thermal efficiency.
Endorsed by scientific opinion, including Lord Kelvin, he won the backing of Krupp and Maschinenfabrik Augsburg.
But years of practical work failed to achieve an isothermal combustion engine, nor could have done, since it requires such an enormous quantity of air that it cannot develop enough power to compress it.
[8] The Carnot heat engine is, ultimately, a theoretical construct based on an idealized thermodynamic system.
On a practical human-scale level the Carnot cycle has proven a valuable model, as in advancing the development of the diesel engine.
However, on a macroscopic scale limitations placed by the model's assumptions prove it impractical, and, ultimately, incapable of doing any work.
[10] For example, for the isothermal expansion part of the Carnot cycle, the following infinitesimal conditions must be satisfied simultaneously at every step in the expansion:[11] Such "infinitesimal" requirements as these (and others) cause the Carnot cycle to take an infinite amount of time, rendering the production of work impossible.
[9] Other practical requirements that make the Carnot cycle impractical to realize include fine control of the gas, and perfect thermal contact with the surroundings (including high and low temperature reservoirs).