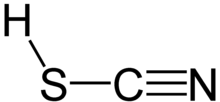Thiocyanic acid
[9] It is a moderately strong acid,[10] with a pKa of 1.1 at 20 °C and extrapolated to zero ionic strength.
[11] One of the thiocyanic acid tautomers, HSCN, is predicted to have a triple bond between carbon and nitrogen.
The esters of thiocyanic acid have the general structure R−S−C≡N, where R stands for an organyl group.
Isothiocyanic acid, HNCS, is a Lewis acid whose free energy, enthalpy and entropy changes for its 1:1 association with a variety of Lewis bases in carbon tetrachloride solution at 25 °C have been reported.
[13]< HNCS acceptor properties are discussed in the ECW model.