Thiostrepton
[citation needed] Thiostrepton was reported (in 2008) to exhibit activity against breast cancer cells through targeting the transcription factor forkhead box M1 (FOXM1),[12] also in 2011.
[14] Thiostrepton is used in molecular biology as a reagent for both positive and negative selection of genes involved in nucleotide metabolism.
[citation needed] Thiostrepton has also shown promise in treating osteoporosis in animal models because it can inhibit unusual osteoclast precursor cells.
Furthermore, amino acid substitutions in the L11 protein that confer resistance to thiostrepton also inhibit the stringent response in strains belonging to the Streptomyces genus.
[19] A similar mechanism has been observed in Neisseria gonorrhoeae, where thiostrepton reduces the synthesis of (p)ppGpp, inhibiting the activation of the persistence.
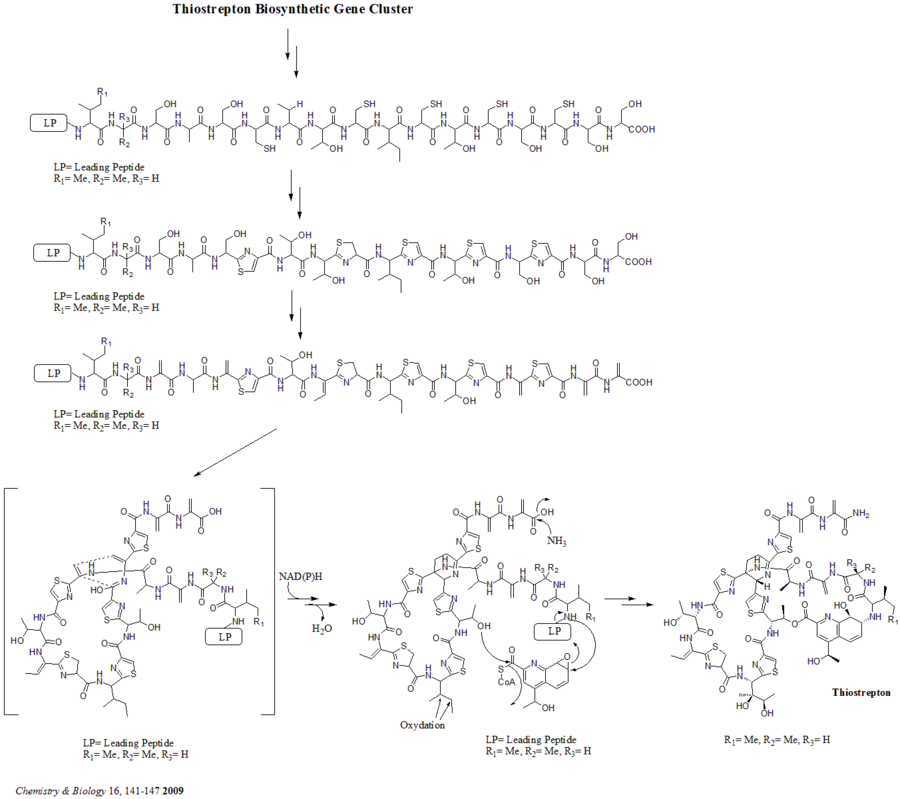
Once the precursor is synthesized, cyclodehydratase tsrO and dehydrogenase tsrM catalyze the formation of thiazole or thiazoline from every cysteine residues in the peptide chain.
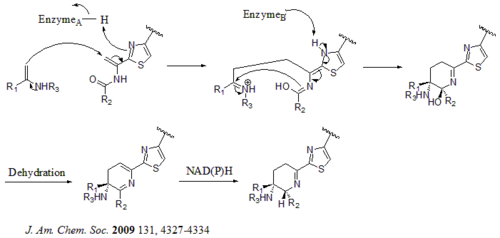
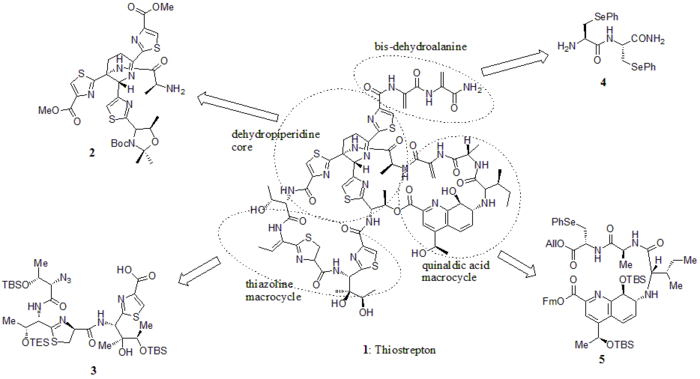
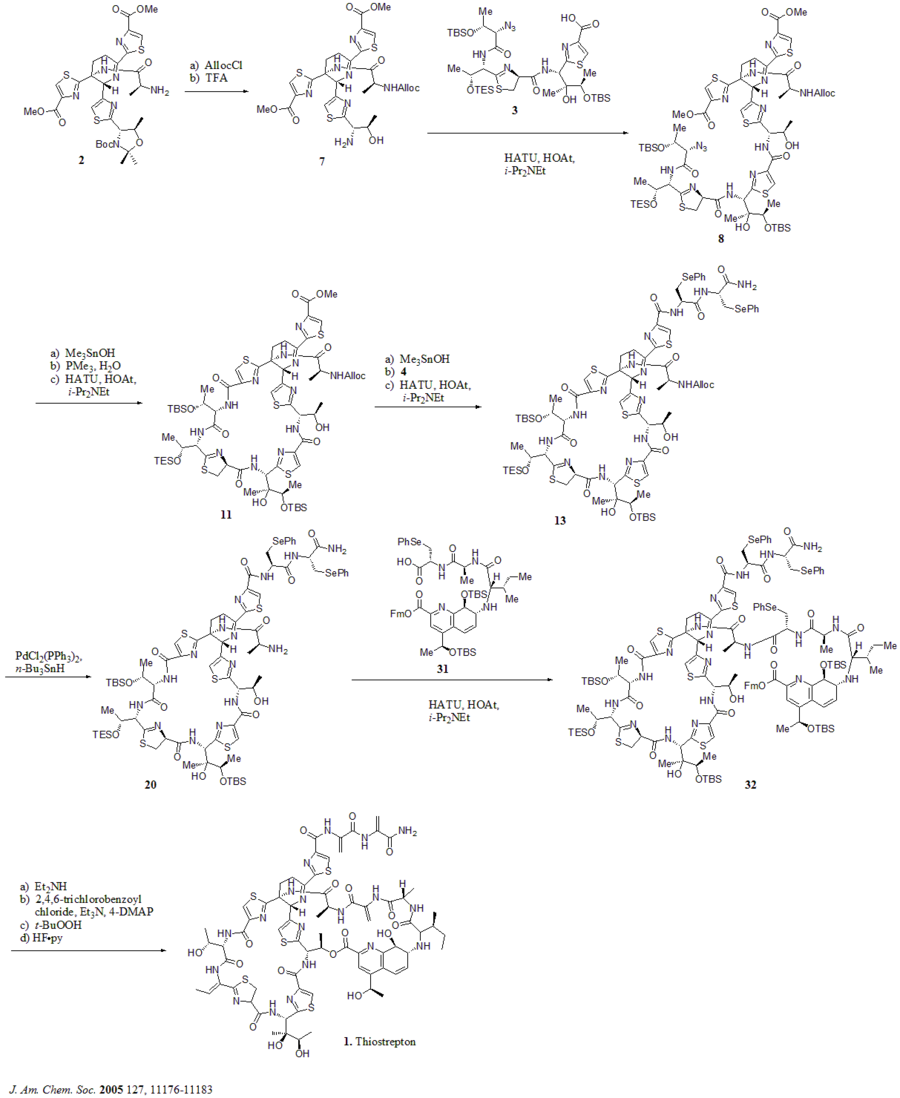
A hetero Diels-Alder cyclization of the central dehydropiperidine (at S5, C13, and S14) has been suggested by Bycroft back to 1978 and been employed in the chemical synthesis of this core structure by Nicolaou et al. in 2005.