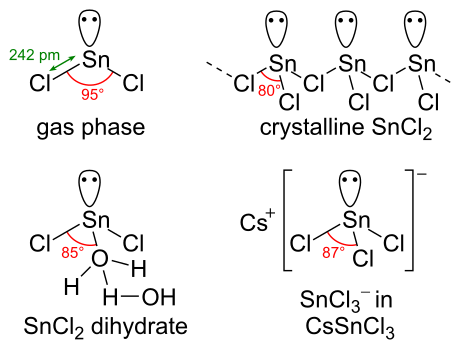
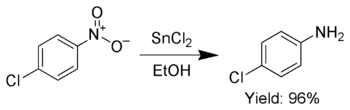
Tin(II) chloride
For example, reaction with sodium sulfide produces the brown/black tin(II) sulfide: If alkali is added to a solution of SnCl2, a white precipitate of hydrated tin(II) oxide forms initially; this then dissolves in excess base to form a stannite salt such as sodium stannite: Anhydrous SnCl2 can be used to make a variety of interesting tin(II) compounds in non-aqueous solvents.
For example, the lithium salt of 4-methyl-2,6-di-tert-butylphenol reacts with SnCl2 in THF to give the yellow linear two-coordinate compound Sn(OAr)2 (Ar = aryl).
For example, the reaction with dicobalt octacarbonyl: Anhydrous SnCl2 is prepared by the action of dry hydrogen chloride gas on tin metal.
In recent years, an increasing number of tooth paste brands have been adding Tin(II) chloride as protection against enamel erosion to their formula, e. g. Oral-B or Elmex.
Stannous chloride is also used by many precious metals refining hobbyists and professionals to test for the presence of gold salts.
[9] When mercury is analyzed using atomic absorption spectroscopy, a cold vapor method must be used, and tin (II) chloride is typically used as the reductant.
In organic chemistry, SnCl2 is mainly used in the Stephen reduction, whereby a nitrile is reduced (via an imidoyl chloride salt) to an imine which is easily hydrolysed to an aldehyde.
A related reaction (called the Sonn-Müller method) starts with an amide, which is treated with PCl5 to form the imidoyl chloride salt.
[12][13] A Stannous reduction is used in nuclear medicine bone scans to remove the negative charge from free pertechnetate when it is bound to MDP for radiopharmaceutical studies.