Tin(II) fluoride
[4] In addition to fluoride, the stannous ion has benefits for oral health when incorporated in a toothpaste.
[17][18] A systematic review revealed stabilised stannous fluoride-containing toothpastes had a positive effect on the reduction of plaque, gingivitis and staining, with a significant reduction in calculus and halitosis (bad breath) compared to other toothpastes.
[21] Aqueous solutions readily oxidise to form insoluble precipitates of SnIV, which are ineffective as a dental prophylactic.
[26] Crystallization from an aqueous solution containing NaF produces compounds containing polynuclear anions, e.g. NaSn2F5 or Na4Sn3F10 depending on the reaction conditions, rather than NaSnF3.
[28] SnF2 is a reducing agent, with a standard reduction potential of Eo (SnIV/ SnII) = +0.15 V.[29] Solutions in HF are readily oxidised by a range of oxidizing agents (O2, SO2 or F2) to form the mixed-valence compound Sn3F8 (containing SnII and SnIV and no Sn–Sn bonds).
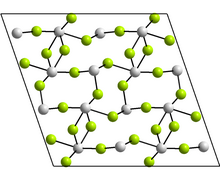
In each case, there are three nearest neighbours, with Sn at the apex of a trigonal pyramid, and the lone pair of electrons sterically active.
[26] Complexes of SnF2, sometimes called difluorostannylene, with an alkyne and aromatic compounds deposited in an argon matrix at 12 K have been reported.
[33] Rare but serious allergic reactions are possible; symptoms include itching, swelling, and difficulty breathing.