Tin(IV) bromide
Tin(IV) bromide is the chemical compound SnBr4.
It is a colourless low melting solid.
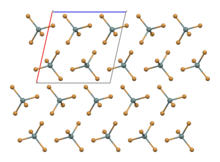
The compound crystallises in a monoclinic crystal system with molecular SnBr4 units that have distorted tetrahedral geometry,[2] with mean Sn-Br bond lengths of 242.3 pm.
[3] SnBr4 can be prepared by reaction of the elements at standard temperature and pressure (STP):[4][page needed] In aqueous solution SnBr4 dissolves to give a series of octahedral (six-ligated) bromo-aquo complexes.
[5] SnBr4 forms 1:1 and 1:2 complexes with ligands, e.g. with trimethylphosphine the following can be produced, SnBr4.P(CH3)3 and SnBr4.2P(CH3)3.