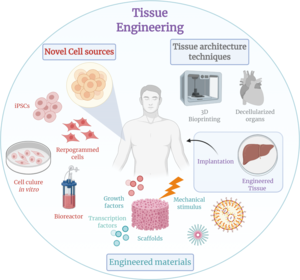
Tissue engineering
Ancient Egyptians often would graft skin from corpses onto living humans and even attempted to use honey as a type of antibiotic and grease as a protective barrier to prevent infection.
In the 17th century, Robert Hooke discovered the cell and a letter from Benedict de Spinoza brought forward the idea of the homeostasis between the dynamic processes in the body.
In 1984, Charles Hull developed bioprinting by converting a Hewlett-Packard inkjet printer into a device capable of depositing cells in 2-D. Three dimensional (3-D) printing is a type of additive manufacturing which has since found various applications in medical engineering, due to its high precision and efficiency.
Today hydrogels are considered the preferred choice of bio-inks for 3-D bioprinting since they mimic cells' natural ECM while also containing strong mechanical properties capable of sustaining 3-D structures.
Whereas digestion processes, typically using enzymes to remove the extracellular matrix (ECM), are required prior to centrifugation or apheresis techniques to extract cells from tissues/organs.
In 2009, an interdisciplinary team led by the thoracic surgeon Thorsten Walles implanted the first bioartificial transplant that provides an innate vascular network for post-transplant graft supply successfully into a patient awaiting tracheal reconstruction.
Protein based materials – such as collagen, or fibrin, and polysaccharidic materials- like chitosan[47] or glycosaminoglycans (GAGs), have all proved suitable in terms of cell compatibility.
[48] The use of thiolated polymers (thiomers) as scaffold material for tissue engineering was initially introduced at the 4th Central European Symposium on Pharmaceutical Technology in Vienna 2001.
[49] As thiomers are biocompatible, exhibit cellular mimicking properties and efficiently support proliferation and differentiation of various cell types, they are extensively used as scaffolds for tissue engineering.
Other than the small thickness range that can be obtained, another drawback of SCPL lies in its use of organic solvents which must be fully removed to avoid any possible damage to the cells seeded on the scaffold.
The main problems resulting from such a technique are caused by the excessive heat used during compression molding (which prohibits the incorporation of any temperature labile material into the polymer matrix) and by the fact that the pores do not form an interconnected structure.
First, a synthetic polymer is dissolved into a suitable solvent (e.g. polylactic acid in dichloromethane) then water is added to the polymeric solution and the two liquids are mixed in order to obtain an emulsion.
By modifying variables such as the distance to collector, magnitude of applied voltage, or solution flow rate – researchers can dramatically change the overall scaffold architecture.
[61] A 2011 study by El-Ayoubi et al. investigated "3D-plotting technique to produce (biocompatible and biodegradable) poly-L-Lactide macroporous scaffolds with two different pore sizes" via solid free-form fabrication (SSF) with computer-aided-design (CAD), to explore therapeutic articular cartilage replacement as an "alternative to conventional tissue repair".
[62] The study found the smaller the pore size paired with mechanical stress in a bioreactor (to induce in vivo-like conditions), the higher the cell viability in potential therapeutic functionality via decreasing recovery time and increasing transplant effectiveness.
[62] In a 2012 study,[63] Koch et al. focused on whether Laser-assisted BioPrinting (LaBP) can be used to build multicellular 3D patterns in natural matrix, and whether the generated constructs are functioning and forming tissue.
[63] Gustafsson et al.[64] demonstrated free‐standing, bioactive membranes of cm-sized area, but only 250 nm thin, that were formed by self‐assembly of spider silk at the interface of an aqueous solution.
The membranes uniquely combine nanoscale thickness, biodegradability, ultrahigh strain and strength, permeability to proteins and promote rapid cell adherence and proliferation.
Engineered tissues generally lack an initial blood supply, thus making it difficult for any implanted cells to obtain sufficient oxygen and nutrients to survive, or function properly.
An ex vivo histological examination showed that certain pore geometry and the pre-growing of chondrocytes (Cho) prior to implantation significantly improves the performance of the created 3-D scaffolds.
In general, the basic requirements of cells must be maintained in culture, which include oxygen, pH, humidity, temperature, nutrients and osmotic pressure maintenance.
Using gene expression analysis, one academic study found that applying a combination of cyclic strain and ultrasound stimulation to pre-osteoblast cells in a bioreactor accelerated matrix maturation and differentiation.
[92] Bioartificial organs are typically created with the intent to restore critical biological functions like in the replacement of diseased hearts and lungs, or provide drastic quality of life improvements like in the use of engineered skin on burn victims.
[96] Integra can be repopulated and revascularized while maintaining its dermal collagen architecture, making it a bioartificial organ[97] Dermagraft, another commercial-made tissue-engineered skin product, is made out of living fibroblasts.
[102] Some of the current TAHs include AbioCor, an FDA-approved device that comprises two artificial ventricles and their valves, and does not require subcutaneous connections, and is indicated for patients with biventricular heart failure.
In vitro and In vivo studies are being conducted to compare and optimize the type of scaffold and to assess whether cell seeding prior to implantation adds to the viability, regeneration and effective function of the kidneys.
[113] In 2018, scientists at Brandeis University reported their research on soft material embedded with chemical networks which can mimic the smooth and coordinated behavior of neural tissue.
[8] The renewed interest in biotechnologies in the 1980s leads to many private investors investing in these new technologies even though the business models of these early startups were often not very clear and did not present a path to long term profitability.
[117] After regulatory changes in 2014, which allowed cell cultivation outside of a hospital setting, the speed of research in Japan increased and Japanese companies also started to develop their own products.
[117] In the 2010s the regulatory framework also started to facilitate faster time to market especially in the US as new centres and pathways were created by the FDA specifically aimed at products coming from living cells such as the Center for Biologics Evaluation and Research.