Titanium diselenide
In this material selenium is viewed as selenide (Se2−) which requires that titanium exists as Ti4+.
Many exhibit properties of potential value in battery technology, such as intercalation and electrical conductivity, although most applications focus on the less toxic and lighter disulfides, e.g. TiS2.
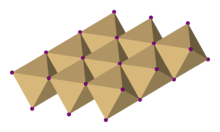
Titanium diselenide crystallizes with the CdI2 -type structure, in which the octahedral holes between alternating hexagonal closely packed layer of Se2− layers (that is half of the total number of octahedral holes) are occupied by Ti4+ centers.
The structure type is found commonly for many transition metal halides as well.
[2] A mixture of titanium and selenium are heated under argon atmosphere to produce crude samples.