Strontium titanate
Its most important application has been in its synthesized form wherein it is occasionally encountered as a diamond simulant, in precision optics, in varistors, and in advanced ceramics.
[2] Other than its type locality of the Murun Massif in the Sakha Republic, natural tausonite is also found in Cerro Sarambi, Concepción department, Paraguay; and along the Kotaki River of Honshū, Japan.
[3][4] Synthetics are usually transparent and colourless, but can be doped with certain rare earth or transition metals to give reds, yellows, browns, and blues.
Strontium titanate is considered extremely brittle with a conchoidal fracture; natural material is cubic or octahedral in habit and streaks brown.
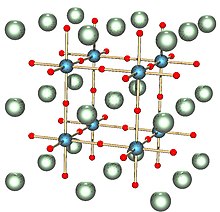
Its bulk lattice parameter of 3.905Å makes it suitable as the substrate for the growth of many other oxides, including the rare-earth manganites, titanates, lanthanum aluminate (LaAlO3), strontium ruthenate (SrRuO3) and many others.
At high temperatures (>200 °C), the main effects of light are photoionic, meaning that they involve the migration of oxygen vacancies (negative ions) in the material.
Research was conducted primarily at the National Lead Company (later renamed NL Industries) in the United States, by Leon Merker and Langtry E. Lynd.
The extra oxygen is required for successful formation of strontium titanate, which would otherwise fail to oxidize completely due to the titanium component.
The salt is washed to eliminate chloride, heated to 1000 °C in order to produce a free-flowing granular powder of the required composition, and is then ground and sieved to ensure all particles are between 0.2 and 0.5 micrometres in size.
The height of the pedestal is constantly adjusted to keep its top at the optimal position below the flame, and over a number of hours the molten powder cools and crystallises to form a single pedunculated pear or boule crystal.
Its cubic structure and high dispersion once made synthetic strontium titanate a prime candidate for simulating diamond.
Strontium titanate was in competition with synthetic rutile ("titania") at the time, and had the advantage of lacking the unfortunate yellow tinge and strong birefringence inherent to the latter material.
Under the microscope, gemmologists distinguish strontium titanate from diamond by the former's softness—manifested by surface abrasions—and excess dispersion (to the trained eye), and occasional gas bubbles which are remnants of synthesis.
[19][20][21] Due to its high melting point and insolubility in water, strontium titanate has been used as a strontium-90-containing material in radioisotope thermoelectric generators (RTGs), such as the US Sentinel and Soviet Beta-M series.
However, due to the lower power density (~0.45W thermal per gram of Strontium-90-Titanate) and half life, space based applications, which put a particular premium on low weight, high reliability and longevity prefer Plutonium-238.
In this case, the A-site, or position in the unit cell where strontium usually sits, is sometimes filled by lanthanum instead, this causes the material to exhibit n-type semiconductor properties, including electronic conductivity.
This material has a thermal coefficient of expansion similar to that of the common electrolyte yttria-stabilized zirconia (YSZ), chemical stability during the reactions which occur at fuel cell electrodes, and electronic conductivity of up to 360 S/cm under SOFC operating conditions.
This material also shows mixed ionic and electronic conductivity which is important as it means the reduction reaction which happens at the cathode can occur over a wider area.