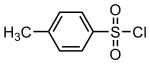
4-Toluenesulfonyl chloride
This white, malodorous solid is a reagent widely used in organic synthesis.
[2] Abbreviated TsCl or TosCl, it is a derivative of toluene and contains a sulfonyl chloride (−SO2Cl) functional group.
The preparation of tosyl esters and amides are conducted in the presence of a base, which absorbs hydrogen chloride.
Unusual bases are also used; for example, catalytic amounts of trimethylammonium chloride in the presence of triethylamine is highly effective by virtue of the trimethylamine.
[2] In an unusual reaction focusing on the sulfur center, zinc reduces TsCl to the sulfinate, CH3C6H4SO2Na.