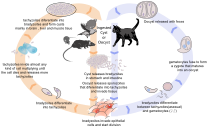Toxoplasma gondii
[3] Found worldwide, T. gondii is capable of infecting virtually all warm-blooded animals,[4]: 1 but felids are the only known definitive hosts in which the parasite may undergo sexual reproduction.
[13][4] T. gondii can initially cause mild, flu-like symptoms in the first few weeks following exposure, but otherwise, healthy human adults are asymptomatic.
[14][15][16] Behavioral changes observed between infected and non-infected humans include a decreased aversion to cat urine (but with divergent trajectories by gender) and an increased risk of schizophrenia.
[30]: 39 Inside these intestinal cells, the parasites undergo sexual development and reproduction, producing millions of thick-walled, zygote-containing cysts known as oocysts.
[32] Humans can be exposed to oocysts by, for example, consuming unwashed vegetables or contaminated water, or by handling the feces (litter) of an infected cat.
[4]: 46 An exhibit at the San Diego Natural History Museum states urban runoff with cat feces transports Toxoplasma gondii into the ocean, which can kill sea otters.
[32] In 2006, researchers reviewed evidence that T. gondii has an unusual population structure dominated by three clonal lineages called Types I, II and III that occur in North America and Europe, despite the occurrence of a sexual phase in its life cycle.
[48] Like tachyzoites, merozoites divide quickly and are responsible for expanding the population of the parasite inside the cat's intestine before sexual reproduction.
Major challenges associated with the ability to cultivate presexual and sexual stages of T. gondii in vitro have limited our understanding of this developmental program and how it is triggered by the parasite in response to the infection of the cat.
[51] However, linking gene expression patterns to stage transitions and deciphering the genetic triggers driving the switch from asexual to sexual development remain unresolved.
Farhat and colleagues [52] showed that chromatin modifiers MORC and HDAC3 play critical roles in silencing sexual development-specific genes.
When a human or other warm-blooded host consumes an oocyst, sporozoites are released from it, infecting epithelial cells before converting to the proliferative tachyzoite stage.
One study of >1600 individuals found that Toxoplasma infection was especially common among people who expressed certain MHC alleles (HLA-B*08:01, HLA-C*04:01, HLA-DRB 03:01, HLA-DQA*05:01 and HLA-DQB*02:01).
[38] The IFN-γ-mediated activation of IDO and TDO is an evolutionary mechanism that serves to starve the parasite, but it can result in depletion of tryptophan in the brain of the host.
"The seawater in California is thought to be contaminated by T. gondii oocysts that originate from cat feces, survive or bypass sewage treatment, and travel to the coast through river systems.
[74] Basic food-handling safety practices can prevent or reduce the chances of becoming infected with T. gondii, such as washing unwashed fruits and vegetables, and avoiding raw or undercooked meat, poultry, and seafood.
As these can spread and survive in the environment for months, humans should wear gloves when gardening or working with soil, and should wash their hands promptly after disposing of cat litter.
Pregnant women are at higher risk of transmitting the parasite to their unborn child and immunocompromised people of acquiring a lingering infection.
[87][88][89][90][91] A 2011 study of 161 Pacific Northwest marine mammals ranging from a sperm whale to harbor porpoises that had either become stranded or died found that 42 percent tested positive for both T. gondii and S.
[85] Approximately 14 per cent of the western Arctic beluga whale population is believed to asymptomatically carry T. gondii with a few deaths attributed to the infection.
Researchers have found that the oocytes of T. gondii can survive in seawater for at least six months, with the amount of salt concentration not affecting its life cycle.
[104] The pathogen can be released either by lysis of the amoebae or by exocytosis, but this is understudied [105] Almost all species of birds that have been tested for Toxoplasma gondii have shown to be positive.
It is suggested that fecal matter from litter boxes be collected daily, placed in a sealable bag, and disposed of in the trash rather than flushed in the toilet, so that water contamination is limited.
The new genus name Toxoplasma is a reference to its morphology: Toxo, from Greek τόξον (toxon, 'arc, bow'), and πλάσμα (plasma, 'shape, form') and the host in which it was discovered, the gundi (gondii).
[119] The first conclusive identification of T. gondii in humans was in an infant girl delivered full term by Caesarean section on May 23, 1938, at Babies' Hospital in New York City.
[7] This is an example of the extended phenotype concept, that is, the idea that the behaviour of the infected animal changes in order to maximize survival of the genes that increase predation of the intermediate rodent host.
It is also important to mention that in 2016 a population-representative birth cohort study which was done, to test a hypothesis that toxoplasmosis is related to impairment in brain and behaviour measured by a range of phenotypes including neuropsychiatric disorders, poor impulse control, personality and neurocognitive deficits.
Thus, according to this study, the presence of T. gondii antibodies is not correlated to increase susceptibility to any of the behaviour phenotypes (except possibly to a higher rate of unsuccessful attempted suicide).
The team notes that the null findings might be a false negative due to low statistical power because of small sample sizes but against this weights that their setup should avoid some possibilities for errors in the about 40 studies that did show a positive correlation.
In July 2024, a study published in Nature Microbiology showed that T. gondii can be engineered to deliver the MECP2 protein, a therapeutic target of Rett syndrome, to the brain of infected mice.