Tramadol
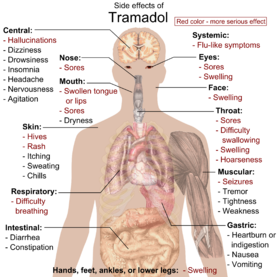
[12] Serious side effects may include hallucinations, seizures, increased risk of serotonin syndrome, decreased alertness, and drug addiction.
[12][19] Tramadol was patented in 1972 and launched under the name "Tramal" in 1977 by the West German pharmaceutical company Grünenthal GmbH.
People with specific variants of CYP2D6 enzymes may not produce adequate amounts of the active metabolite (desmetramadol) for effective pain control.
[32] A small prospective study in France found, while an increased risk of miscarriages existed, no major malformations were reported in the newborn.
[23] The most common adverse effects of tramadol include nausea, dizziness, dry mouth, indigestion, abdominal pain, vertigo, vomiting, constipation, drowsiness, and headache.
[40] The clinical presentation in overdose cases can vary but typically includes neurological, cardiovascular, and gastrointestinal manifestations.
[45][46] Additionally, individuals with genetic variations leading to CYP2D6 enzyme duplication (rapid metabolizers) may have an increased risk of adverse effects, due to faster conversion of tramadol to its active metabolite.
These include common antiarrhythmics, antiemetics, antidepressants (sertraline, paroxetine, and fluoxetine in particular),[57] antipsychotics, analgesics, and tamoxifen.
[58] Due to tramadol's serotonergic effects, tramadol has the potential to contribute to the development of an acute or chronic hyper-serotonin state called serotonin syndrome when used concurrently with other pro-serotonergic medications such as antidepressants (SSRIs, SNRIs, tricyclics, MAOIs), antipsychotics, triptans, cold medications containing dextromethorphan, and some herbal products such as St. John's wort.
[68][69] In addition, a few studies have found that it also acts as a serotonin releasing agent (1–10 μM), similar in effect to fenfluramine.
[99][91] A positron emission tomography imaging study found that single oral 50-mg and 100-mg doses of tramadol to human volunteers resulted in 34.7% and 50.2% respective mean occupation of the serotonin transporter (SERT) in the thalamus.
[102] The estimated median effective dose (ED50) for SERT occupancy hence was 98.1 mg, which was associated with a plasma tramadol level of about 330 ng/mL (1,300 nM).
[24][103] Some accumulation of tramadol occurs with chronic administration; peak plasma levels with the maximum oral daily dosage (100 mg q.i.d.)
[24] Positron emission tomography imaging studies have reportedly found that tramadol levels are at least four-fold higher in the brain than in plasma.
[19][106] However, other studies have found that the analgesic effects of tramadol are significantly decreased or even absent in CYP2D6 poor metabolizers.
[24][108] Pharmacologically, tramadol is similar to tapentadol and methadone in that it not only binds to the MOR, but also inhibits the reuptake of serotonin and norepinephrine[11] due to its action on the noradrenergic and serotonergic systems, such as its "atypical" opioid activity.
Antagonism of 5-HT2C could be partially responsible for tramadol's reducing effect on depressive and obsessive–compulsive symptoms in patients with pain and co-morbid neurological illnesses.
Thus, 2-(dimethylaminomethyl)-1-(3-methoxyphenyl)cyclohexanol may exist in four different configurational forms: The synthetic pathway leads to the racemate (1:1 mixture) of (1R,2R)-isomer and the (1S,2S)-isomer as the main products.
[116] Tramadol and desmetramadol may be quantified in blood, plasma, serum, or saliva to monitor for abuse, confirm a diagnosis of poisoning or assist in the forensic investigation of a sudden death.
Most commercial opiate immunoassay screening tests do not cross-react significantly with tramadol or its major metabolites, so chromatographic techniques must be used to detect and quantify these substances.
[117][118][119] In 2013, researchers Michel de Waard (then at Université Joseph Fourier, Grenoble and Grenoble Institute of Neuroscience, La Tronche[120]) reported in Angewandte Chemie that tramadol was found in relatively high concentrations (>1%) in the roots of the African pin cushion tree, Nauclea latifolia, concluding that it was a natural product in addition to its being a later human synthetic, and presenting a putative biosynthetic hypothesis for its origin.
[120] They further observed that tramadol and its mammalian metabolites were found in tree roots in the far north of Cameroon where the commercial drug was in use, but not in the south where it was not being administered.
[122][better source needed] In 2016, Spiteller and colleagues followed up their preceding work with a radiocarbon analysis that supported their contention that the tramadol found in N. latifolia roots was of human synthetic origin rather being plant-derived.
[23] The U.S. Food and Drug Administration (FDA) approved tramadol in March 1995, and an extended-release (ER) formulation in September 2005.
[128] Effective August 2014, tramadol has been placed into Schedule IV of the federal Controlled Substances Act in the United States.
[23] Effective May 2008, Sweden classified tramadol as a controlled substance in the same category as codeine and dextropropoxyphene, but allows a normal prescription to be used.
[134] In June 2014, the United Kingdom's Home Office classified tramadol as a Class C, Schedule 3 controlled drug, but exempted it from the safe custody requirement.
[7] In October 2023, New Zealand's Medsafe reclassified tramadol as a Class C2 Controlled Drug (in addition to its existing status as a prescription only medication).
[139] It is also abused in the United Kingdom, inspiring the title of the TV show Frankie Boyle's Tramadol Nights (2010).
[142][143] Tramadol may be used to treat post-operative, injury-related, and chronic (e.g., cancer-related) pain in dogs and cats as well as rabbits, coatis, many small mammals including rats and flying squirrels, guinea pigs, ferrets, and raccoons.