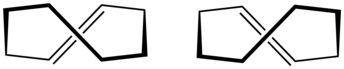
trans-Cyclooctene
Computations indicate that the most stable "crown" conformation has the carbon atoms alternately above and below the plane of the ring.
[5] All conformations of trans-cyclooctene are chiral (specifically, what some call planar-chiral[6]) and the enantiomers can be separated.
[7][8][9] In theory, conversion of between the enantiomers can be done, without breaking any bonds, by twisting the whole –CH=CH– group, rigidly, by 180 degrees.
[7] trans-Cyclooctene was first synthesized on a preparatory scale by Arthur C. Cope with a Hofmann elimination reaction of N,N,N-trimethylcyclooctylammonium iodide.
For instance, it can be prepared in almost 100% yield by converting the cis isomer to 1,2-epoxycyclooctane ("cyclooctene oxide") followed by reactions with lithium diphenylphosphide (LiPPh2) and with methyl iodide CH3I.