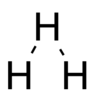Triatomic hydrogen
In the early universe this ability to emit infrared light allowed the primordial hydrogen and helium gas to cool down so as to form stars.
[4] H+3 ions can be made in a duoplasmatron where an electric discharge passed through low pressure molecular hydrogen.
If the molecule attempts to lose energy and go to the repulsive ground state, it spontaneously breaks up.
[8] The reason that earlier observers could not see any H3 spectral lines, was due to them being swamped by the spectrum of the much more abundant H2.
[1] Transitions dropping to the lower 2s2A1' state are affected by its very short lifetime in what is called predissociation.
It is believed to have played a crucial role in the cooling of early stars in the history of the Universe through its ability readily to absorb and emit photons.
He stated that the easiest way to make it was to target potassium hydroxide with cathode rays.
[8] In 1913 Johannes Stark proposed that three hydrogen nuclei and electrons could form a stable ring shape.
In 1919 Niels Bohr proposed a structure with three nuclei in a straight line, with three electrons orbiting in a circle around the central nucleus.
[8] In 1916 Arthur Dempster showed that H3 gas was unstable, but at the same time also confirmed that the cation existed.
[8] Joseph Lévine went so far as to postulate that low pressure systems on the Earth happened due to triatomic hydrogen high in the atmosphere.
[8] In 1920 Wendt and Landauer named the substance "Hyzone" in analogy to ozone and its extra reactivity over normal hydrogen.
[12] Earlier Gottfried Wilhelm Osann believed he had discovered a form of hydrogen analogous to ozone which he called "Ozonwasserstoff".
This idea was later proven with the existence of tritium, but that was not the explanation of why molecular weight 3 was observed in mass spectrometers.
[13] In the Orion nebula lines were observed that were attributed to nebulium which could have been the new element eka-hydrogen, especially when its atomic weight was calculated as near 3.