Triboracyclopropenyl
They are the lightest and smallest cyclic structures known to display the bonding and magnetic properties that originate from fully delocalized electrons in orbitals of σ and π symmetry.
Although three-membered rings of boron are frequently so highly strained as to be experimentally inaccessible, academic interest in their distinctive aromaticity and possible role as intermediates of borane pyrolysis motivated extensive computational studies by theoretical chemists.
[1][2][3][4] Beginning in the late 1980s with mass spectrometry work by Anderson et al. on all-boron clusters, experimental studies of triboracyclopropenyls were for decades exclusively limited to gas-phase investigations of the simplest rings (ions of B3).
[4][5][6] However, more recent work has stabilized the triboracyclopropenyl moiety via coordination to donor ligands or transition metals, dramatically expanding the scope of its chemistry.
[7][8][9][10] For gas-phase spectroscopic studies, triboracyclopropenyl-containing compounds are obtained via laser ablation of boron targets and collimation of the resulting plasma cloud in a flow of inert carrier gas such as helium.
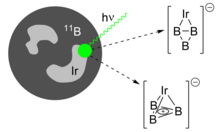
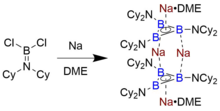
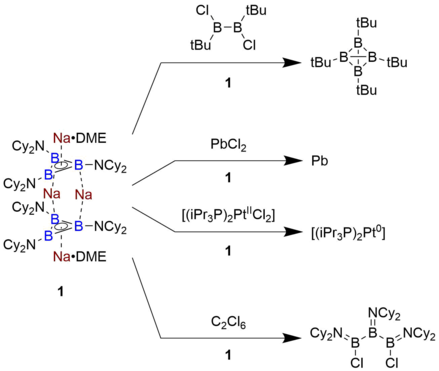
[11] The sole isolable example of a triboracyclopropenyl anion that persists in solution and in the solid state was identified by Braunschweig and coworkers, who synthesized it by reducing the aminoborane Cl2B=NCy2 (Cy = cyclohexyl) with finely dispersed sodium metal in dimethoxyethane (DME).
[12] Due to their special status as the simplest aromatic cycles, the electronic structure of triboracyclopropenyl derivatives has been analyzed with a variety of techniques in computational chemistry.
[15] In general, the extremely small size of these cycles implies that their bonding electrons experience substantial Coulomb repulsion, resulting in abnormally high ring strain.
[17] ETS-NOCV energy decomposition analysis suggests that the N2 and CO adducts are primarily stabilized (by -83.6 and -112.3 kcal/mol respectively) through σ donation of the exocyclic ligands into the highly electron-deficient boron ring.
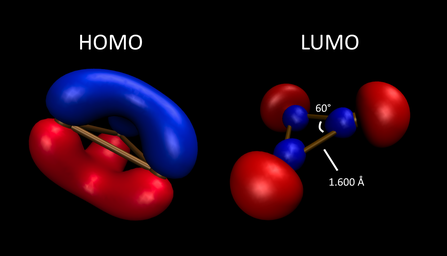
[19][21] B3 possesses a singly occupied a1' HOMO (a SOMO) that consists of σ-symmetric orbitals oriented toward the core of the ring, associated with σ delocalization and slightly shorter B-B bond lengths as compared to B3+.
8 electrons are assigned to the triboracyclopenyl core, 6 in σ bonding orbitals and 2 in the π system, resulting in Hückel aromaticity.
[6] Subsequently, B3 was isolated in matrices of frozen noble gases and electron paramagnetic resonance spectra were recorded which confirmed its D3h geometry.
This level of reducing power is roughly comparable to an alkali metal, and has not been previously observed for any molecule based on an organic framework.
[7] Although most examples of transition metal-doped trinuclear boron clusters do not contain an aromatic triboracyclopropenyl fragment, the reactivity of such species with small molecules is likely to attract increasing scientific interest.