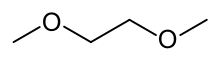
Dimethoxyethane
Monoglyme is produced industrially by the reaction of dimethylether with ethylene oxide:[3][4] Together with a high-permittivity solvent (e.g. propylene carbonate), dimethoxyethane is used as the low-viscosity component of the solvent for electrolytes of lithium batteries.
Dimethoxyethane is often used as a higher-boiling-point alternative to diethyl ether and tetrahydrofuran.
Dimethoxyethane acts as a bidentate ligand for some metal cations.
Grignard reactions and hydride reductions are typical application.
Sodium naphthalide dissolved in dimethoxyethane is used as a PTFE etching solution that removes fluorine atoms from the surface, which get replaced by oxygen, hydrogen, and water.