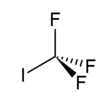
Trifluoroiodomethane
[3] The mechanism of extinguishing fires for CF3I is active and primarily based on interruption of the chain reaction in the combustion area of the flame by so-called "negative" catalytic action.
[4] It is also used as an eco-friendly insulation gas to replace SF6 in electrical power industry.
[citation needed] Trifluoroiodomethane contains carbon, fluorine, and iodine atoms.
Although iodine is several hundred times more efficient at destroying stratospheric ozone than chlorine, experiments have shown that because the weak C-I bond breaks easily under the influence of water (owing to the electron-attracting fluorine atoms), trifluoroiodomethane has an ozone depleting potential less than one-thousandth that of Halon 1301 (0.008-0.01).
Its atmospheric lifetime, at less than 1 month, is less than 1 percent that of Halon 1301, and less even than hydrogen chloride formed from volcanoes.