Trifluoroperacetic acid
[5][7] In September 1953, the Journal of the American Chemical Society published work by William D. Emmons and Arthur F. Ferris reporting that this reagent, generated in situ, was capable of oxidising aniline to nitrobenzene.
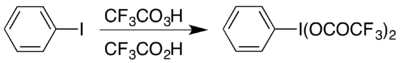
One example is the formation of the hypervalent iodine compound (bis(trifluoroacetoxy)iodo)benzene, (CF3COO)2IC6H5 which is used to carry out the Hofmann rearrangement under acidic conditions.
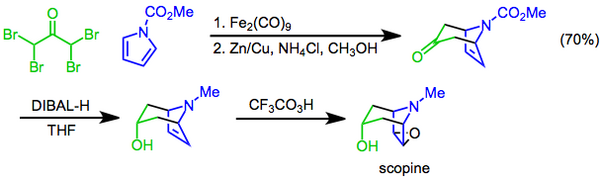
In this approach, a [4+3] cycloaddition mediated by diiron nonacarbonyl is used to construct the bicyclic skeleton, the hydroxyl functional group is then introduced by diastereoselective reduction of the ketone with diisobutylaluminum hydride, and the preparation completed with a Prilezhaev trifluoroperacetic acid epoxidation.
[2] In the case of an allyl alcohol compound with a proximate carbonyl functional group, the epoxide can undergo a ring-expansion reaction to form a dioxolane.
[5][11] The process below was used as part of the total synthesis of neosporol, a natural product:[11][25] The preparation of the isomeric compound sporol involved a similar dioxolane formation.
In this case, the use of trifluoroperacetic acid derived from hydrogen peroxide, which therefore presumably contained traces of water, gave mostly a hemiacetal rather than the closed-ring dioxolane.
[5] In the analogous selenium system, trifluoroperacetic acid oxidation of selenoethers (R–Se–R) produces selones (R–Se(O)2–R) without the formation of the related selenoxides (R–Se(O)–R) as an isolable product,[3] a reaction which is particularly effective when the R is an aryl group.
[31][Note 2] The major pathway initially forms the sulfoxide, but this chemical promptly undergoes a Diels-Alder-type dimerisation before any further oxidation occurs—neither thiophene-S-oxide or thiophene-S,S-dioxide are found among the products of the reaction.
[31] The choice of trifluoroperacetic acid preparation method is important as water suppresses the minor reaction pathway, likely because it acts as a competing base.
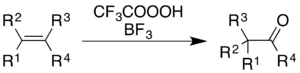
[31] The use of trifluoroperacetic acid with boron trifluoride causes oxidation of alkenes and aromatic rings with concomitant rearrangement of the molecular skeleton.
[5] For alkenes, the reaction gives a ketone product, though the mechanistic process is not simply epoxidation followed by a BF3-catalyzed Wagner–Meerwein rearrangement:[36] For aromatics, an example demonstrated in an Organic Syntheses report is the conversion of hexamethylbenzene to 2,3,4,5,6,6-hexamethyl-2,4-cyclohexadienone:[9] In addition to simple oxidation of aromatic rings to form carbonyl compounds (see § Oxidation with acidic rearrangement), trifluoroperacetic acid can fully cleave the carbon–carbon bonds within the ring.


6 H
5 I(OOCCF
3 )
2